Review Article - Imaging in Medicine (2010) Volume 2, Issue 2
Ultrasound and its role in assisted reproduction treatment
Kannamannadiar Jayaprakasan, Shilpa Deb, Shyamaly Sur, Po‑Mui Lam, Milhan Batcha, Nicola Porter, Beverley Winter, Jeanette Clewes and Nick Raine‑Fenning†
Division of Human Development, School of Clinical Sciences, University of Nottingham, Nottingham, NG7 2UH, UK
- *Corresponding Author:
- Nick Raine‑Fenning
Academic Imaging, Nottingham University Research & Treatment Unit in Reproduction (NURTURE)
Division of Human Development, School of Clinical Sciences
University of Nottingham, Nottingham
NG7 2UH, UK
Tel: +44 115 823 0700
Fax:+44 115 823 0651
E-mail:nick.fenning@ nottingham.ac.uk
Abstract
Ultrasound has become an essential tool for the assessment and management of women undergoing assisted reproduction treatment. Ultrasound permits the screening of women prior to treatment and prediction of ovarian reserve and endometrial receptivity, allows for direct monitoring of response to controlled ovarian stimulation, and facilitates oocyte retrieval and embryo transfer. While 3D ultrasound and recently introduced software, such as sonographic automated volume count, have the potential to improve the workflow of fertility units, their use is currently limited in the research setting. This article critically evaluates the available evidence in an attempt to establish the current role of both conventional and advanced ultrasound in the management of women undergoing assisted reproduction treatment.
Keywords
assisted reproduction treatment; endometrial receptivity; in vitro fertilization; ovarian reserve ultrasound; pelvic pathology; pretreatment; screening
Ultrasound is an essential tool for the assessment and management of women undergoing assisted reproduction treatment (ART). A baseline ultrasound assessment of the pelvis prior to ART is performed to screen for any pelvic pathologies that are known to have a negative effect on fertility and early pregnancy. The other key areas that the pretreatment assessment facilitates include the prediction of the woman’s response to ovarian stimulation (ovarian reserve), identification of ovarian position with respect to accessibility for oocyte retrieval and evaluation of the chance of implantation of an embryo (endometrial receptivity). Furthermore, ultrasound allows for the direct monitoring of response to controlled ovarian stimulation, and facilitates oocyte retrieval and embryo transfer (ET). This article critically evaluates the available evidence in an attempt to establish the current role of both conventional and advanced ultrasound in the management of women undergoing ART.
Assessment of pelvic pathology
Polycystic ovarian syndrome
The most common cause of medically treatable infertility is polycystic ovarian syndrome (PCOS), which is the cause of anovulatory infertility in 70% of cases [1] and, therefore, is an important condition to diagnose in the assessment of infertile couples. The diagnosis of PCOS not only carries important long-term health implications, such as increased metabolic risks, but also helps to guide the ART. Women with PCOS are characterized by an increased ovarian responsiveness to gonadotrophins and, therefore, have a higher risk of ovarian hyperstimulation syndrome [2], which is associated with significant morbidity and even mortality [3]. An accurate diagnosis of PCOS allows the clinician to formulate an optimal ovarian stimulation protocol aiming for a multifollicular response without the complications of ovarian hyperstimulation syndrome [4].
Despite its importance, the diagnosis of PCOS has been a source of controversy for a long time. During the first international conference on PCOS at the NIH in USA in 1990, three key features of PCOS were generally agreed: chronic anovulation, hyperandrogenism (clinical or laboratory evidence) and the absence of other endocrine disorders (e.g., congenital adrenal hyperplasia, hyperprolactinemia or thyroid abnormalities) [5]. This first consensus identified the core group of PCOS subjects with the most significant features of the disease and the greatest metabolic risks. However, it did not address the heterogeneity of the syndrome where the associated clinical features, such as menstrual disturbance, obesity and hyperandrogenism, manifesting as hirsutism or acne, vary considerably between women [6]. Furthermore, the appearance of polycystic ovaries on ultrasonography was not included in the definition, despite this feature being mandatory in many centers [7]. Therefore, all subsequent definitions of PCOS, both the 2003 Rotterdam criteria [8] and the 2006 Androgen Excess Society (AES) criteria [9], expand the diagnosis beyond the NIH definition by adding the sonographic appearance of polycystic ovaries and allowing some flexibility in the diagnosis [8,9].
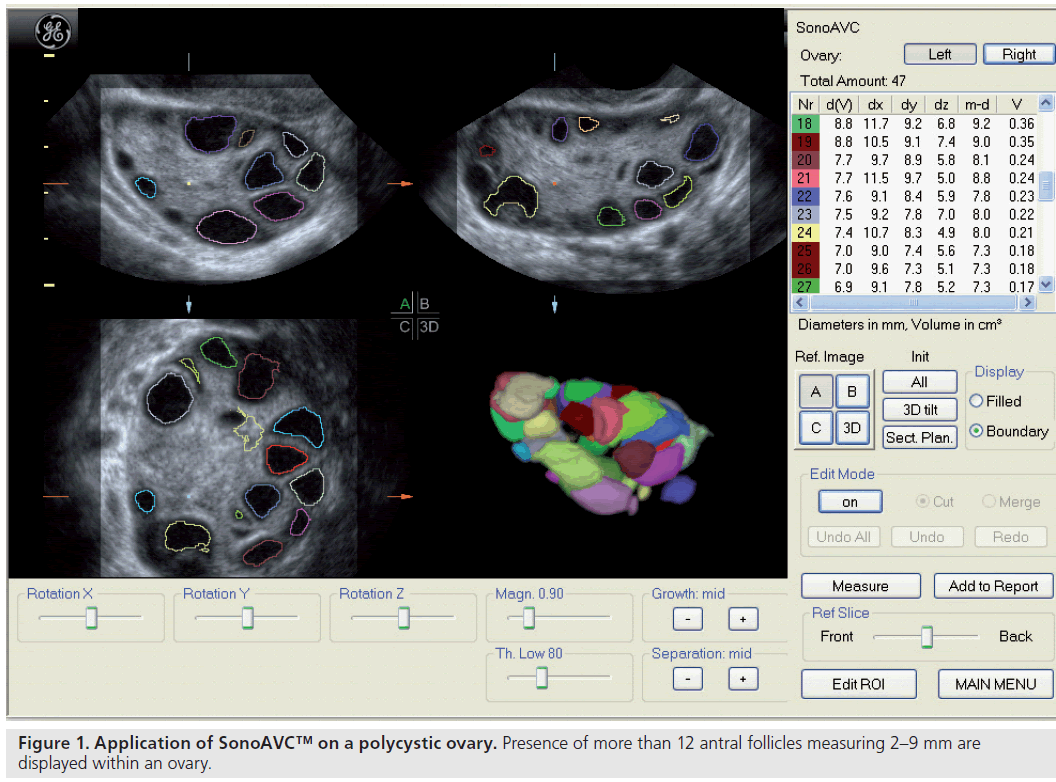
Since the advent of ultrasound, numerous parameters have been proposed to morphologically define PCOS, but there have been no consensus regarding their diagnostic values. The classical image is that of enlarged ovaries containing an increased number of small follicles encircling the ovarian cortex together with an increased bright echogenic stroma [10]. In the recent Rotterdam criteria, PCOS is defined as either 12 or more follicles measuring 2–9 mm in diameter or an increased ovarian volume greater than 10 cm3 (Figure 1) [11], while the distribution of follicles and a description of the stroma are not included. However, the evaluation of ovarian stroma by ultrasound could be too subjective or too difficult to apply in routine daily practice.
In the Rotterdam guidelines, the calculation of ovarian volume is based on geometric assumptions from 2D measurements. A simplified formula for a prolate ellipsoid (0.5 × length × width × thickness) is used, which assumes the ovary to be ovoid, although many polycystic ovaries are irregular in shape. More importantly, this geometric assumption cannot be applied to the irregularly-shaped ovarian stroma. Instead, the stromal:area (S:A) ratio, which is defined as the ovarian stromal area outlining the peripheral profile of the central hyperechoic stroma divided by the total area of the ovary in its maximum plane section, have been suggested to indirectly evaluate the stromal hypertrophy and to correlate with serum androgen levels and fasting insulin levels [12]. A cut-off value of 0.32 for the S:A ratio appears to be the best predictor of elevated serum androstenedione and testosterone levels. The S:A ratio is subjective and only evaluates a single plane of the ovary, but these data at least suggest that the stroma may be important in women with PCOS.
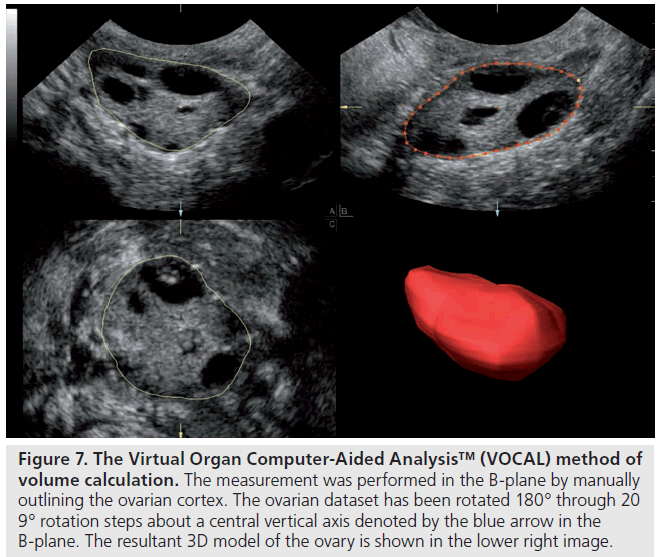
Volume estimations are more reliable and valid when made using 3D ultrasound [13,14]. There are various ways to derive volume measures but the rotational method through the Virtual Organ Computer-Aided Analysis™ (VOCAL)-imaging program is commonly used. 3D ultrasound also permits a unique measurement of stromal volume through the calculation and subtraction of total follicular volume from total ovarian volume [15].
With the conventional 2D ultrasound scan, the degree of echogenicity of the ovarian stroma is usually assessed subjectively and this is open to observer bias, especially when other features of PCOS have already been seen. With the advances in ultrasound software, the echogenicity of the ovarian stroma can be determined objectively by measuring the intensity level of the ultrasound pixels within the stroma displayed on the sonographic image. The mean echogenicity of a given area can then be calculated. While the degree of stromal echogenicity does not differ significantly between women with PCOS and controls, the stromal index, defined as the ratio of mean stromal echogenicity to mean echogenicity of the entire ovary, is significantly higher in women with PCOS [16]. These findings are thought to relate to the increased volume of ovarian stroma relative to a lower mean echogenicity of the entire ovary due to an increased number of follicles. 3D ultrasound provides an alternative way to examine stromal echogenicity objectively through the assessment of the mean grayness of the ovary, which represents the mean tissue intensity or echogenicity in the region of interest [15]. Current evidence using 3D ultrasound demonstrates no differences in stromal echogenicity measured between the polycystic ovary and control groups.
The apparent stromal hypertrophy seen in women with PCOS may relate to increased vascularity within the ovarian stroma. Ovarian vascularity can be assessed subjectively through the application of color or power Doppler maps to a single plane to examine the flow pattern or can be assessed objectively by measuring flow velocity and the resistance to flow through pulsed-wave Doppler on a single vessel. It has been observed that the pulsatility index and resistance index of both ovarian artery and stromal artery were significantly lower in women with PCOS [17], but this was not supported by other investigators [18]. Quantitative 3D power Doppler angiography facilitates the assessment of total blood flow within the defined volume of interest, allowing the objective assessment of total vascular flow within an organ or a specified volume of tissue [19]. The data on ovarian blood flow quantified using 3D power Doppler in a polycystic ovary are conflicting [20,21].
Ovarian cysts
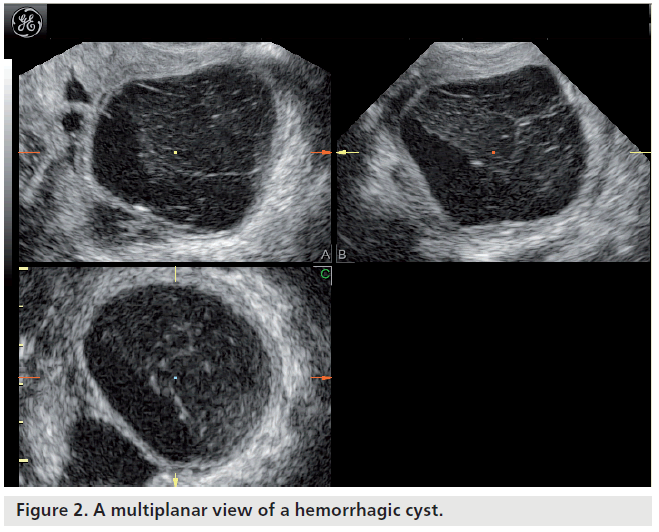
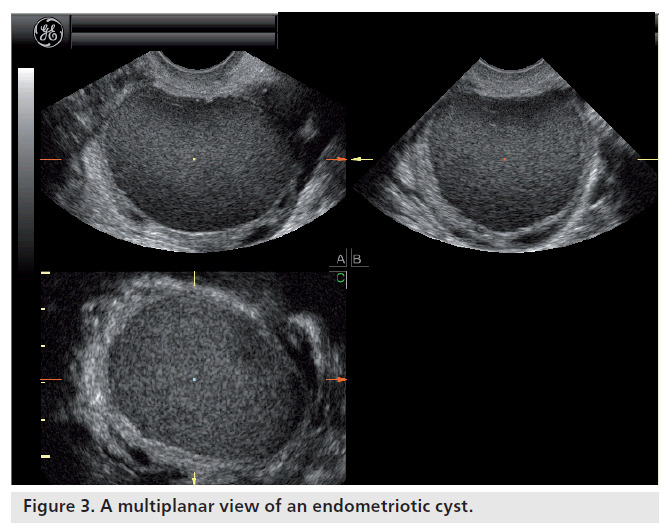
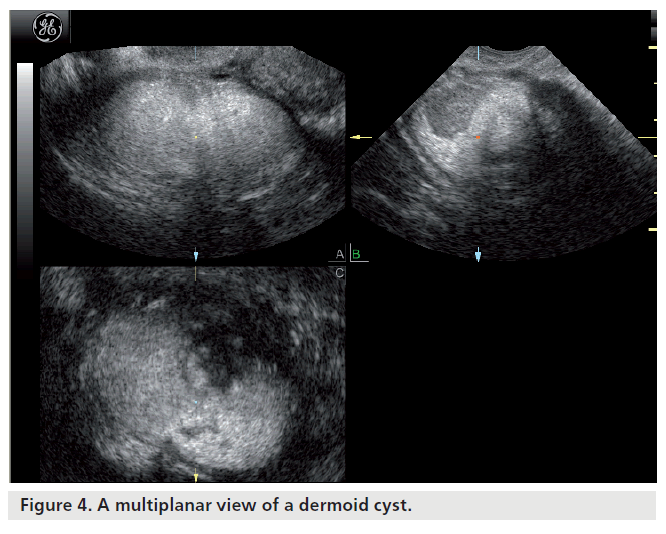
Most ovarian cysts in women of reproductive age are benign in nature with characteristic ultrasonographic appearances. Ovarian cysts are readily diagnosed with transvaginal ultrasound, which offers a sensitivity of 88–100% and specificity of 62–96% in the discrimination between benign and malignant adnexal masses [22]. Many ultrasonographic misdiagnoses relate to the difficulty in differentiating between endometriotic, dermoid and hemorrhagic cysts [23]. Hemorrhagic cysts have diffuse low-level echoes, reflective of the fibrinous strands and the retracting clots they contain, and are readily recognizable through pattern recognition (Figure 2). An adnexal mass with diffuse low-level internal echoes (‘ground glass’ appearance) is highly likely to be an endometrioma (Figure 3), especially if multilocularity and hyperechoic wall foci are also present, but 8% of endometriomata demonstrate similar patterns to hemorrhagic cysts. By contrast, dermoid cysts demonstrate areas of focal acoustic impedance in association with bright echoes and hyperechoic lines and dots (Figure 4). A distinctive feature is the presence of a discrete, highly echogenic focus with posterior shadowing (Rokitansky protuberance). Other characteristics considered pathognomonic of a dermoid cyst include fine, echogenic bands representing hair within the cystic area and the presence of a fat-fluid level. Although 3D techniques have the potential to improve the diagnostic accuracy of ultrasound for differentiating between these benign ovarian cysts, through the additional spatial information provided by the multiplanar view, tomographic ultrasound imaging and different rendering modalities, they need to be tested in prospective studies [23].
Most benign ovarian cysts do not have a direct negative effect on a woman’s fertility but their management may have implications, especially in subfertile patients. The excision of an ovarian cyst measuring more than 5 cm, to reduce the risk of torsion and rupture, is common practice among many gynecologists. Cystectomy, however, may have a detrimental effect on ovarian reserve as surgery necessitates a division of the ovarian cortex and often involves electrodiathermy to achieve hemostasis. Ovarian cystectomy has been demonstrated to impair ovarian reserve [24,25] and ovarian response [26,27] following ART; however, this does not seem to translate into impaired pregnancy outcome [28]. Few studies have reported contradictory results, wherein ovarian cystectomy does not influence ovarian response to controlled stimulation [29]. In the absence of definitive evidence, differentiation of benign and malignant cysts is important and surgery should not be routinely offered for benign cysts unless the patient is symptomatic.
Hydrosalpinx
In women undergoing in vitro fertilization (IVF), the presence of a hydrosalpinx is associated with early pregnancy loss and poor implantation and pregnancy rates [30]. The exact effect of hydrosalpinges on fertility is unknown but they are thought to produce substances toxic to the endometrium that negatively affect endometrial receptivity [31]. Salpingectomy, ideally performed laparoscopically, prior to IVF has been demonstrated to be beneficial and is recommended [30]. In technically difficult cases caused by adhesions, tubal occlusion by clip application may also be effective.
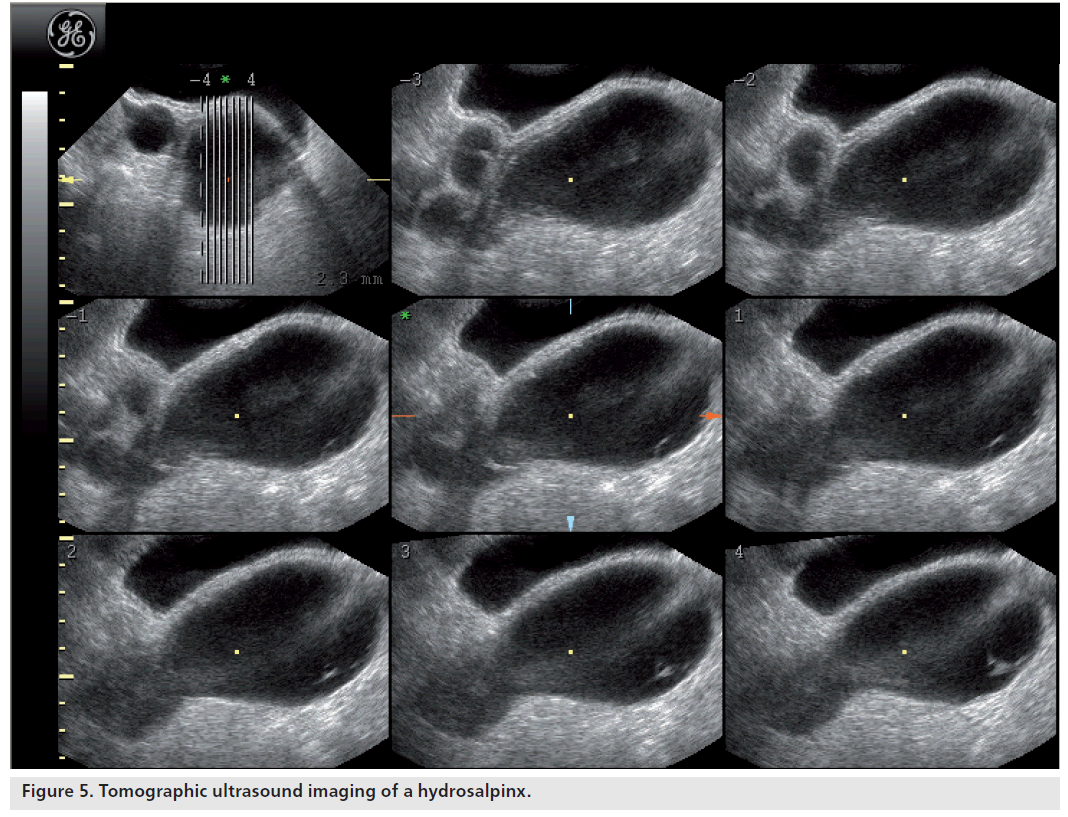
The diagnosis of a hydrosalpinx using transvaginal ultrasound can generally be made with a high degree of confidence. The sensitivity and specificity of transvaginal ultrasound in the diagnosis of hydrosalpinx are 84.6 and 99.7%, respectively, with positive and negative predictive values of 91.7 and 99.4%, respectively [22]. The typical appearance of a hydrosalpinx is of a fluid-filled, sausage-shaped, cystic structure with incomplete hyperechoic septa. The ‘beads-on-a-string’ sign describes the presence of hyperechoic mural nodules measuring approximately 2–3 mm, which are seen on cross-sections of the fluid-filled distended structure. They may be mistaken as a collapsing ovarian cyst or a paraovarian cyst. Recently introduced 3D tomographic ultrasound imaging can help differentiate between these as it provides a series of consecutive images similar to the displays seen with MRI and CT (Figure 5).
Figure 5.Tomographic ultrasound imaging of a hydrosalpinx.
Congenital uterine anomalies
It has been demonstrated that congenital uterine anomalies are more common in infertile women and in those with recurrent miscarriage as compared with the general population [32,33]. The precise classification of a uterine anomaly is of clinical importance as it carries prognostic signiicance with respect to obstetric and gynecologic complications, such as miscarriage or preterm labor [34,35], and it critically defines the need and feasibility of any intervention. Diagnostic laparoscopy and hysteroscopy were considered the gold standard but they do not allow a simultaneous view of the external and internal contour of the uterus and have certain operative and anesthetic risks. Therefore, hysterosalpingography or 2D ultrasound [36] are often used, however, the former can only assess the internal uterine contour and the latter is only sensitive for major anomalies and neither can reliably differentiate the various types of congenital uterine anomalies. Sonohysterography is highly valuable as it provides additional information to conventional ultrasound in evaluating the uterine cavity and myometrium, and could be applied routinely as a first-line, office-based diagnostic tool [37]. The quality of assessment can be improved further with the use of 3D ultrasound. 3D pelvic ultrasonography provides a reconstructed coronal plane, which allows the evaluation of both the external and internal contour of the uterus at the same time. This technique has been shown to be reliable in diagnosing and differentiating various congenital uterine anomalies and in the conformation of normality as it is also specific [38,39]. MRI can also evaluate the external and internal contour but is considerably more expensive and largely reserved for complex anomalies not readily classifiable with 3D ultrasound [40].
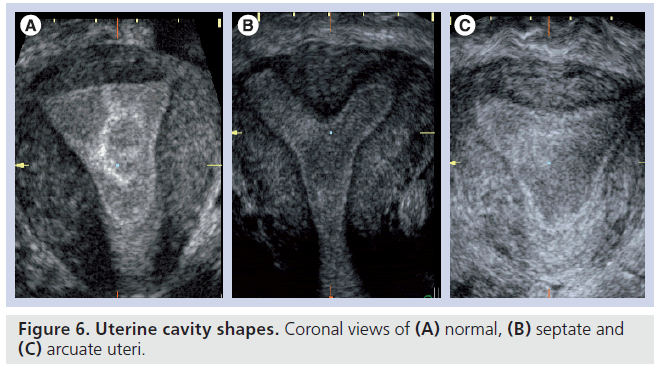
The double endometrial echo complex seen on a transverse plane with 2D ultrasound is indicative of a uterine anomaly but this is observed with arcuate, septate and bicornuate uteri. A transverse slice through the fundus of an arcuate uterus can give the view of a double endometrial echo complex, although this mildest form of uterine anomaly, with a shallow internal fundal indentation of 1 cm or less, is of doubtful clinical importance [41]. Nevertheless, a bicornuate uterus, which involves indentation in the external fundal contour by 1 cm or more, is of clinical significance as it is associated with increased risks of miscarriage and preterm labor but is not surgically treatable. By contrast, the septate uterus has a smooth external fundal contour or a shallow external fundal indentation of less than 1 cm in depth, but has a similar internal fundal indentation, of at least 1 cm in depth, to the bicornuate uterus, and demonstrates similar risks of miscarriage and preterm labor. Most importantly, this anomaly is correctable by hysteroscopic resection of the uterine septum, which has been demonstrated to provide improvements in reproductive outcomes [42]. The 3D coronal view can be used to not only objectively measure the width and the length of uterine septum but also the length of the remaining ‘normal’ uterine cavity, which has been demonstrated to have the closest relationship with aberrant pregnancy outcome (Figure 6) [43].
The unicornuate uterus is harder to identify with conventional imaging as it does not give the classical double endometrial echo complex on 2D ultrasound. Although it is not correctable, it is associated with a high risk of miscarriage and preterm labor [41] and it is clinically important to identify so that patients can be appropriately counseled and identified as high risk in any pregnancy.
Other uterine pathologies
Fibroids can be difficult to define clearly as they are often homogenous and blend in with the surrounding myometrial tissue from which they are derived. However, the classification of fibroids into subserosal, intramural or submucosal is usually straightforward and important, as these progressively exert a negative effect on pregnancy rates, respectively. While intramural fibroids measuring more than 4 cm in diameter and submucous fibroids of any size are thought to negatively affect the outcome of fertility treatment and to be associated with lower pregnancy rates, the effect that smaller intramural fibroids have on reproductive outcome is unclear [44]. A recent meta-analysis of 19 observational studies has concluded that noncavity-distorting intramural fibroids may adversely affect IVF pregnancy outcome regardless of its size, although it is unclear whether removal of such fibroids will improve the outcome [45]. There is also doubt regarding what exact impact endometrial polyps, the other commonly encountered intrauterine pathology, have on treatment outcome and early pregnancy. Some authors suggest conservative management, especially if it is small [46], but this can be difficult in patients undergoing fertility treatment or with a history of miscarriage when a polyp is identified before treatment begins. Polyps are usually evident with conventional transvaginal imaging, although false-positive diagnoses are common. Sonohysterography can reduce this and facilitate appropriate operative planning [47]. Delineation of the polyp with saline ensures that the size and position of the polyp can be accurately defined allowing surgeons to modify their approach and resect the larger, more broad-based polyps rather than list them for simple polypectomy.
Assessment of ovarian reserve
Ovarian reserve has emerged as an important concept in the field of reproductive medicine, especially in the treatment of infertility. It is defined as the existent quantitative and qualitative supply of follicles in the ovaries that can potentially develop into mature follicles, which, in effect, determines a woman’s reproductive potential. It is also used as a term to determine the capacity of the ovary to provide oocytes that are capable of fertilization, resulting in a healthy and successful pregnancy. Reproductive aging is thought to be due to a gradual decrease in both the quantity and quality of the oocytes contained within the follicles present in the ovarian cortex [48]. The fecundity declines with a women’s age and this change, secondary to the decrease in oocyte quality and quantity, is significant after 37 years of age. Natural population studies have demonstrated that by a mean age of 41 years, the natural fecundity has already reached its nadir [49]. This age-related decline in fertility is also seen in couples undergoing ARTs (2003 CDC ART report) [101]. A poor response to ovarian stimulation in such treatment programs is a strong predictor of nonconception, spontaneous fecundity and early menopause [50,51]. As women postpone childbearing, there has been an expected increase in the incidence of subfertility due to ovarian aging. Evaluation of ovarian reserve has become increasingly important as it allows couples to be counseled and allows individualization of treatment protocols for those requiring assisted reproduction. The commonly employed tests of ovarian reserve can be divided into static markers (estradiol, follicle stimulating hormone, inhibin‑B and anti-Mullerian hormone), dynamic markers (tests of stimulation with clomiphene citrate, gonadotrophins and gonadotrophin-releasing hormone analog) and ultrasonographic markers (antral follicle count [AFC], ovarian volume and ovarian blood flow).
Ovarian volume
The first ultrasound marker of ovarian reserve to be evaluated was ovarian volume. It is easily determined and can be calculated from 2D images, using the principle of the volume of an ellipsoid using the formula: length × width × depth × p/6, or with 3D ultrasound (Figure 7) [13], which seems to provide a more reliable and valid method of volume calculation. The role of ovarian volume as a marker of reserve is unclear. Syrop et al. found a reduction in estradiol levels, the number of oocytes collected and pregnancy rates correlated with decreasing ovary volume in women undergoing assisted reproduction cycles [52] and Bowen et al. found a significant correlation between reduced ovarian volume, increasing age and follicle-stimulating hormone levels [53]. Other studies have not seen significant differences in ovarian volume between normal and poor responders in women aged less than 37 years [54] or in women at high risk for cancellation of assisted reproduction cycles [55]. Schild et al. examined the role of ovarian volume measurements made using pretreatment 3D datasets in 152 IVF cycles and found no relation to IVF outcome other than a nonsignificant trend to smaller ovarian measurements in the conception group [56]. Subgroup analysis comparing absolute ovarian size revealed a pregnancy rate of only 6.7% (one out of 15) in patients with a minimum unilateral ovarian volume of no more than 3 cm3, which represented a single standard deviation below the mean, compared with 21.9% (30 out of 137) in patients with a minimum ovarian volume of more than 3 cm3. In a recent systematic review, Broekmans et al. concluded that ovarian volume has little clinical application in the prediction of poor pregnancy response [57].
Figure 7.Virtual Organ Computer-Aided Analysis™ (VOCAL) method of volume calculation. The measurement was performed in the B-plane by manually outlining the ovarian cortex. The ovarian dataset has been rotated 180° through 20 9° rotation steps about a central vertical axis denoted by the blue arrow in the B-plane. The resultant 3D model of the ovary is shown in the lower right image.
Antral follicle count
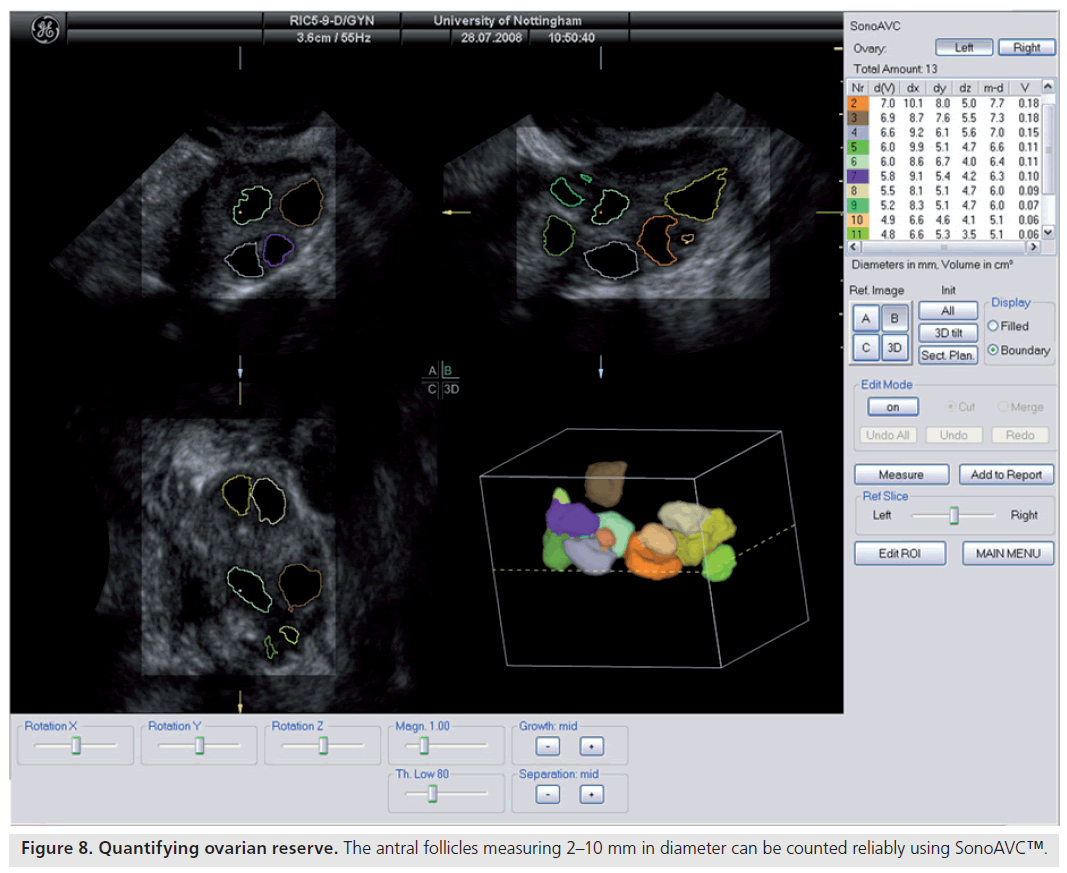
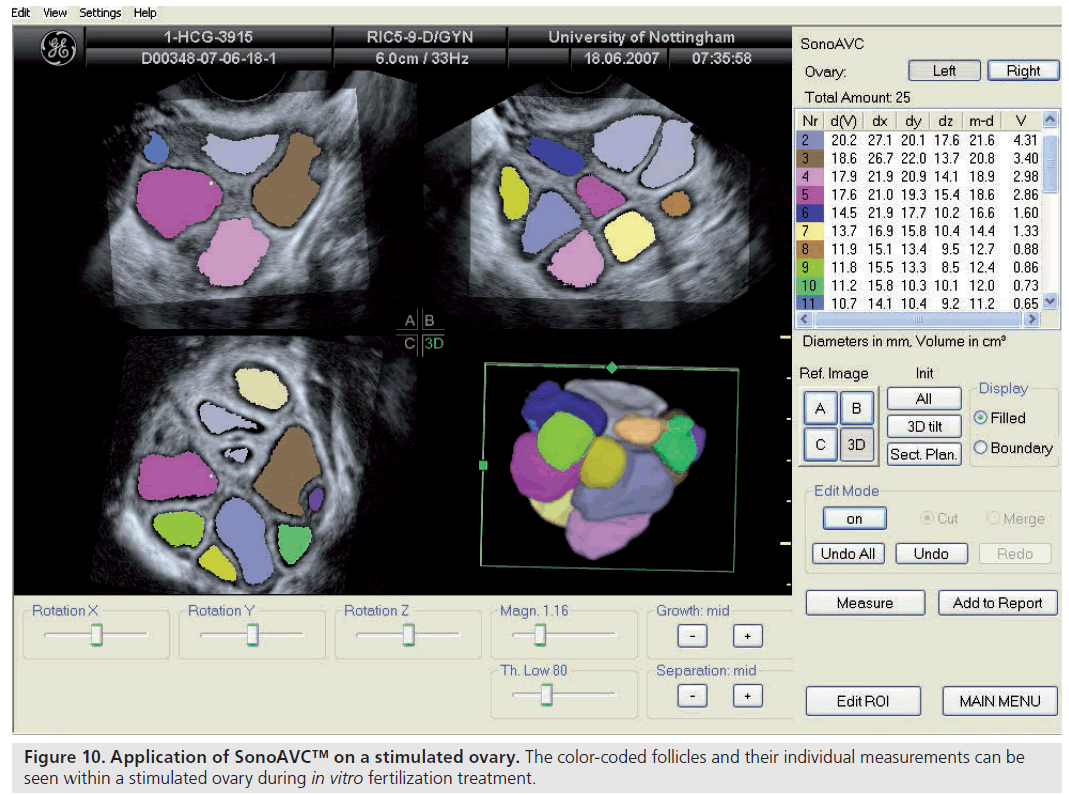
Ultrasonographic assessment of the total number of antral follicles measuring 2–10 mm is a reliable determinant of ovarian reserve [58,59]. The total AFC is performed by counting the number of antral follicles measuring 2–10 mm in both ovaries and can be estimated using 2D [60,58] or 3D ultrasound [61]. 3D ultrasound has two main advantages: allowing display of an image in three perpendicular planes, simultaneously giving more spatial orientation, and offline assessment of data along with the facility to render the image. 3D ultrasound has recently been demonstrated to offer a significant advantage over 2D imaging in terms of measurement reliability [58]. Recent studies evaluating various ovarian reserve markers and response have demonstrated that the AFC may be considered as the test of choice in the assessment of diminished ovarian reserve [62]. Recent developments have seen the introduction of a 3D automated technique; Sonography-based Automated Volume Count™ (SonoAVC; GE Healthcare Ultrasound, Zipf, Austria) [63], which allows a semi-automated analysis of the antral follicle population (Figure 8) [64].
Ovarian blood flow
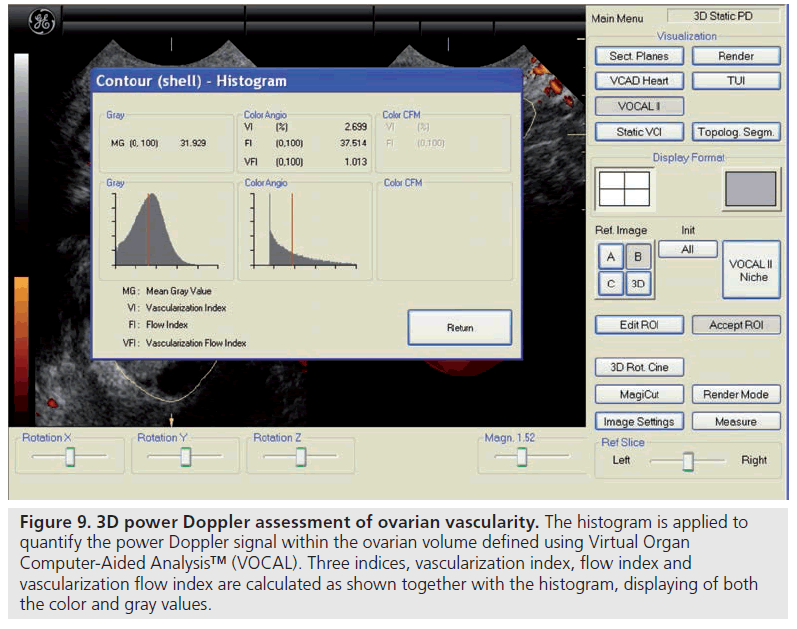
There are only a few studies reporting on the predictive capacity of ovarian vascular flow parameters for ovarian response or the occurrence of pregnancy [65–69]. Kupesic et al. used 3D power Doppler angiography (Figure 9) to evaluate ultrasound-derived ovarian predictors of ovarian response and outcome in 56 women with normal basal serum follicle-stimulating hormone concentrations (<10 mIU/ml) undergoing their first cycle of IVF [67]. Multiple regression analysis of the six most predictive variables – including the peak estradiol on the day of human chorionic gonadotropin administration, total ovarian volume, total ovarian stromal area, age and total AFC – revealed AFC to be the best predictor of the number of mature oocytes collected and pregnancy, followed by the ovarian stromal flow index, which is a measure of the intensity of blood flow. Similar findings were reported in larger subsequent studies by Ng et al. and Jayaprakasan et al., in which the AFC achieved the best predictive value in relation to the number of oocytes retrieved, although the 3D ovarian vascular indices were not predictive of ovarian response or pregnancy [68,69]. Despite these studies, very few centers use ovarian blood flow clinically and it essentially remains a research tool.
Figure 9.3D power Doppler assessment of ovarian vascularity. The histogram is applied to quantify the power Doppler signal within the ovarian volume defined using Virtual Organ Computer-Aided Analysis™ (VOCAL). Three indices, vascularization index, flow index and vascularization flow index are calculated as shown together with the histogram, displaying of both the color and gray values.
Follicular monitoring
Follicular tracking describes the serial ultrasonographic study of the ovary during the follicular phase of the menstrual cycle. The aim is to estimate follicular maturity, so that the time of ovulation can be predicted, or to enable interventions to be appropriately timed during ART [70].
During follicular tracking, the mean diameter of each of the follicles is measured and their growth followed. Ultrasound is often used in conjunction with serum measures of estradiol, luteinizing hormone and progesterone to improve accuracy.
Follicular development in the normal menstrual cycle
A cohort of small antral follicles develops during the luteal phase of previous menstrual cycles independently of gonadotrophin stimulation. Further development of the follicle is only possible in the presence of appropriate levels of gonadotrophin, whereby the most mature follicle is selected and the others become atretic. The leading follicle grows at approximately 2 mm per day until ovulation, which occurs at a mean diameter of 20–25 mm [70]. At the time of ovulation, meiosis II resumes, leading to the formation of a mature oocyte capable of fertilization, loss of the germinal vesicle and extrusion of the first polar body. Ovulation is characterized by a blurring of the follicular borders, the appearance of intrafollicular echoes and the appearance of free fluid in the pouch of Douglas. Following ovulation, the corpus luteum may be seen, which can appear as a thicker and more irregular walled cystic structure with circumferential blood flow referred to as a ‘ring of fire’. The corpus luteum has a limited life expectancy and resolves if pregnancy does not occur at the end of the luteal phase. Rarely, ovulation fails to occur leading to a luteinized, unruptured follicle in which, despite the absence of follicular rupture and release of the oocyte, the unruptured follicle undergoes luteinization under the action of luteinizing hormone. Ultrasound, but not the midluteal progesterone levels, aids in diagnosing this condition as there is normal production of progesterone.
When is follicular tracking used?
Follicular tracking is important during controlled ovarian stimulation and used as part of IVF treatment when multifollicular recruitment is desired. A large number of oocytes are recruited for fertilization to provide a pool of embryos from which the best can be selected for transfer into the endometrial cavity and any excess good quality ones are frozen to increase the cumulative conception rates. Ultrasound is again used to ensure adequate stimulation is occurring and to identify patients at risk of ovarian hyperstimulation as well as to time final follicular maturation, which is essential prior to oocyte retrieval to induce the resumption of meiosis. Mature oocytes are generally retrieved from follicles measuring at least 14 mm in mean diameter and most IVF centers aim to carry out oocyte retrieval when there are three or more follicles measuring 17–18 mm or more. Wittmaack et al. demonstrated that the highest oocyte recovery rates (83.5%) were achieved with follicles measuring 3–4 ml, which is the equivalent of a mean follicular diameter of between 18 and 20 mm [71] while cleavage rates may be higher (92%) in follicles measuring 6–7 ml, which equates to a mean diameter of between 23 and 24 mm. Nataprawira et al. also demonstrated that while follicular diameter correlated well with fluid volume, the number of mature oocytes and fertilization rates were higher when an oocyte was retrieved from a follicle measuring between 5 and 7 ml than when obtained from follicles with a volume of between 1 and 5 ml [72].
Follicle size, both in terms of diameter and volume, can be estimated using 2D and 3D ultrasound. Recently introduced software, SonoAVC, has been used to provide automated measurements of follicle size from the stored 3D datasets (Figure 10). While this technique appears promising and may have implications for the work flow within an IVF center [73], timing final follicle maturation and oocyte retrieval on the basis of such automated measures does not appear to improve the clinical outcome of ART [74].
Embryo transfer
Embryo transfer is a crucial step in IVF treatment. In most IVF centers, ET is done under ultrasound guidance. However, the evidence to support this ultrasound guidance is conflicting. A recent systematic review of randomized control trials has suggested ultrasound-guided ET significantly increases the chance of live birth and ongoing and clinical pregnancy rates compared with the clinical touch method [75]. ET under ultrasound involves a transabdominal scan of the uterus in its longitudinal axis. The ultrasound transducer is manipulated as the embryo catheter is inserted to ensure the catheter tip is identified and followed as the catheter is advanced. The couple can witness the transfer of the embryo or embryos through observation of the culture media and associated air bubbles on the ultrasound machine. The air bubbles, which help secure the fluid and embryo(s) within the catheter, are readily evident on ultrasound as a bright, hyperechogenic line or as two small dots. Some catheters are impregnated with air bubbles to facilitate identification of the catheter on ultrasound.
The exact location of the catheter tip can be confirmed by sweeping the ultrasound probe along the longitudinal axis of the uterus or by acquiring a 3D dataset. The best place to transfer the embryo(s) is also subject to debate. A randomized control trial has shown that pregnancy rates are higher when the embryos are released 1.5–2 cm away from the fundus [76]. However, Franco et al. found no differences in implantation or pregnancy rates when comparing embryos transferred in the upper or lower half of the endometrial cavity [77], and two recent studies have also failed to demonstrate any advantage with ultrasound guidance [78,79]. The important point seems to be to avoid touching the fundus with the catheter tip, which may induce uterine contractions and potentially be detrimental to implantation or even lead to ectopic pregnancy [80]. However, a meta-analysis by Sallam et al., did not show any significant difference in the incidence of ectopic pregnancy between clinical touch and ultrasound guidance [81].
The angle between the uterine cervix and body can make ET technically difficult. Sallam et al. used transabdominal ultrasound to measure the angle between the cervix and the body of the uterus and then artificially molded the ET catheter to mimic this [82]. This resulted in higher clinical pregnancy and implantation rates and reduced the incidence of difficult and bloody transfers compared with the routine clinical touch method where ultrasound guidance was not used. An acute cervico–uterine angle below 115° appears to be an independent predictor of difficult ET and the need for a more firm but malleable catheter, which is associated with significantly reduced implantation and clinical pregnancy rates.
Whether or not ultrasound is used to predict difficult transfers, guide the passage of the catheter, train new doctors or demonstrate the procedure to the couple, it may still have a role in the general assessment of the woman prior to transfer. The scan provides an opportunity to detect new intrauterine pathology that may arise after oocyte retrieval [83] and also allows reassessment of the patient’s risk of ovarian stimulation. Although ultrasound-guided ET may not have a clear effect on pregnancy rates, there are a number of positive effects of ultrasound-guided ET that cannot be ignored.
Assessment of endometrial receptivity
Endometrial receptivity is the generic term to describe how the endometrium will respond to embryo implantation. The actual contribution of the endometrium to the overall implantation process, relative to that of the embryo, is a matter of conjecture. The endometrium must play some part, however, as conception is unlikely in patients with a thin endometrium and in women who have received pelvic radiotherapy or certain forms of chemotherapy [84]. Several predictive tests of endometrial receptivity have been suggested, of which ultrasound is the most appropriate as it allows a noninvasive but direct assessment of the endometrium and subendometrium.
The majority of centers restrict assessment to the measurement of endometrial thickness and a description of endometrial pattern. Conception is unlikely in association with an endometrial thickness of 5 mm or less, although most units would prefer to see it measure 8 mm or more prior to ET [85,86]. While a recent large study of 1294 IVF cycles confirmed the close relationship between endometrial thickness and pregnancy rate, the authors questioned the not uncommon practice of canceling ET in the presence of a thin endometrium as pregnancy rates of up to 50% were still achieved in women with endometria measuring 6 mm [87].
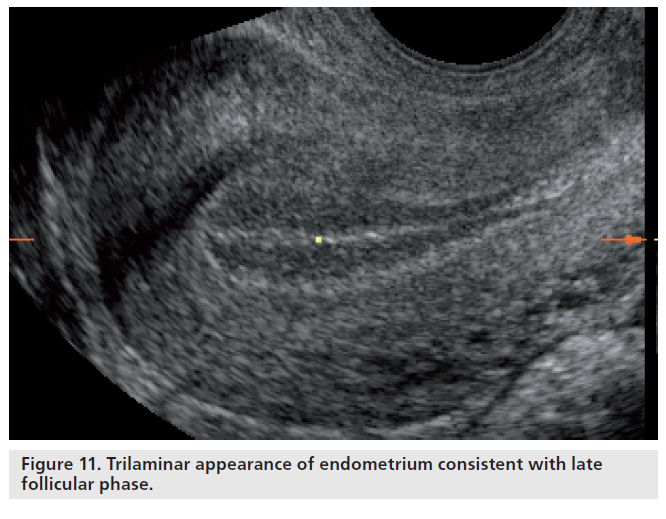
Simple correlation statistics between the various echogenic patterns of the endometrium and endometrial receptivity, assessed by considering implantation and pregnancy as an outcome measure, have repeatedly demonstrated that the trilaminar pattern is more favorable than a prematurely hyperechogenic endometrium on the day of ovulation or administration of human chorionic gonadotrophin to promote final follicular maturation in ART cycles [88]. While a trilaminar pattern is more frequently associated with conception, a homogenous, hyperechogenic endometrium lacking an echogenic central line has also repeatedly been demonstrated to be associated with nonconception in both natural and ART cycles [88,89]. These findings indicate that premature luteinization and the associated rise in progesterone lead to an ‘out-of-phase’ endometrium, which has been shown to be associated with lower pregnancy rates in ART cycles [90]. Nevertheless, endometrial echogenicity and premature rises in progesterone do not show a consistent association with nonconception [91]. The discrepancies may reflect the degree of observer reproducibility of pattern recognition. However, the presence of a trilaminar pattern (Figure 11) does not guarantee a successful outcome even in the presence of high-quality embryos. While advanced endometrial development, as defined as the presence of a homogenously echogenic endometrial pattern on the day of human chorionic gonadotropin administration, has a strong negative predictive value of 85.7% for conception, a trilaminar pattern on the same day only appears to have a positive predictive value of 33.1% and a specificity of 13.7% for the prediction of clinical pregnancy following ART [92].
Adequate endometrial blood flow is generally regarded as a marker of endometrial receptivity [93]. However, reports from studies that correlated endometrial and subendometrial blood flow, as measured by 2D pulse-wave Doppler, with implantation or pregnancy following IVF treatment are conflicting. While some groups suggest blood flow within the spiral arteries as reflected by measurements of resistance to flow (pulsatility index) and absolute velocity (peak systolic velocity) is not predictive of pregnancy [93,94], others report significantly lower spiral artery pulsatility index in pregnant cycles than nonpregnant cycles [95]. A more detailed ultrasound assessment of the endometrium can be made including 3D quantification of endometrial volume, and endometrial and subendometrial blood flow [96]. However, the current evidence is unclear and the current clinical value of these parameters for the prediction of pregnancy following IVF treatment is limited [97].
Uterine contractile activity at the time of implantation is considered detrimental to the endometrial receptivity. Fanchin et al. reported significantly lower pregnancy rates in women (13%) that have an increased uterine contractile activity (five or more contractions per minute) when compared with women (54%) with lower uterine activity (three or less contractions per minute) [98]. Further studies have confirmed this negative association of symmetrical contractions with the chances of pregnancy following ART [99]. The role of uterine contractile activity as a marker of endometrial receptivity warrants further research and, currently, assessment of uterine contraction does not form part of a routine endometrial assessment.
Conclusion
Ultrasound allows a noninvasive, direct assessment of the pelvic organs. It is used daily to monitor the response to treatment and to guide oocyte collection and ET in women undergoing IVF treatment. It is also used as a baseline tool to investigate subfertile women for ruling out any pelvic pathology and for assessment of ovarian reserve, although not universally at present. However, there is sufficient evidence to justify scanning all women prior to treatment as many patients have asymptomatic disease known to reduce the chance of conception if left untreated. The absence of a randomized controlled trial comparing clinical pelvic examination to transvaginal ultrasound does not negate the readily evident fact that the latter is able to detect pelvic pathology more accurately and to quantify it objectively. Therefore, ultrasound should form part of the routine assessment of subfertile women, even in the absence of clinical features suggestive of disease.
Future perspective
Further advances in the ultrasound, specifically with regard to resolution and development of automated software in the coming years, will enhance the objective evaluation of female pelvis. 3D ultrasound and SonoAVC has already proven to improve the workflow of the fertility units. There is a potential for SonoAVC to redefine the ovulation trigger criteria and to improve the ART outcome, although current evidence is limited.

Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
*of interest
* of considerable interest
References
- Hamilton-Fairley D, Taylor A: Anovulation. BMJ 327, 546–549 (2003).
- Agrawal R, Conway G, Sladkevicius P et al.: Serum vascular endothelial growth factor and Doppler blood flow velocities in in vitro fertilization: relevance to ovarian hyperstimulation syndrome and polycystic ovaries. Fertil. Steril. 70, 651–658 (1998).
- Delvigne A, Rozenberg S: Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum. Reprod. Update 8, 559–577 (2002).
- Hart R: PCOS and infertility. Panminerva Med. 50, 305–314 (2008).
- Legro RS: Polycystic ovary syndrome: the new millenium. Mol. Cell Endocrinol. 184, 87–93 (2001).
- Polson DW, Adams J, Wadsworth J, Franks S: Polycystic ovaries – a common finding in normal women. Lancet 1, 870–872 (1988).
- Balen A: Pathogenesis of polycystic ovary syndrome – the enigma unravels? Lancet 354, 966–967 (1999).
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group: Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 19, 41–47 (2004). & Describes the current diagnostic criteria of polycystic ovary syndrome.
- Azziz R, Carmina E, Dewailly D et al.: Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J. Clin. Endocrinol. Metab. 91, 4237–4245 (2006).
- Adams J, Franks S, Polson DW et al.: Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet 2, 1375–1379 (1985).
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group: Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 81, 19–25 (2004).
- Fulghesu AM, Angioni S, Frau E et al.: Ultrasound in polycystic ovary syndrome – the measuring of ovarian stroma and relationship with circulating androgens: results of a multicentric study. Hum. Reprod. 22, 2501–2508 (2007).
- Raine-Fenning NJ, Campbell BK, Clewes JS, Johnson IR: The interobserver reliability of ovarian volume measurement is improved with three-dimensional ultrasound, but dependent upon technique. Ultrasound Med. Biol. 29, 1685–1690 (2003).
- Raine-Fenning NJ, Clewes JS, Kendall NR, Bunkheila AK, Campbell BK, Johnson IR: The interobserver reliability and validity of volume calculation from three-dimensional ultrasound datasets in the in vitro setting. Ultrasound Obstet. Gynecol. 21, 283–291 (2003).
- Lam PM, Johnson IR, Raine-Fenning NJ: Three-dimensional ultrasound features of the polycystic ovary and the effect of different phenotypic expressions on these parameters. Hum. Reprod. 22, 3116–3123 (2007).
- Buckett WM, Bouzayen R, Watkin KL, Tulandi T, Tan SL: Ovarian stromal echogenicity in women with normal and polycystic ovaries. Hum. Reprod. 14, 618–621 (1999).
- Ozkan S, Vural B, Caliskan E, Bodur H, Turkoz E, Vural F: Color Doppler sonographic analysis of uterine and ovarian artery blood flow in women with polycystic ovary syndrome. J. Clin. Ultrasound 35, 305–313 (2007).
- Tugrul S, Oral O, Guclu M, Kutlu T, Uslu H, Pekin O: Significance of Doppler ultrasonography in the diagnosis of polycystic ovary syndrome. Clin. Exp. Obstet. Gynecol. 33, 154–158 (2006).
- Raine-Fenning NJ, Campbell BK, Clewes JS, Kendall NR, Johnson IR: The interobserver reliability of threedimensional power Doppler data acquisition within the female pelvis. Ultrasound Obstet. Gynecol. 23, 501–508 (2004).
- Jarvela IY, Mason HD, Sladkevicius P et al.: Characterization of normal and polycystic ovaries using three-dimensional power Doppler ultrasonography. J. Assist. Reprod. Genet. 19, 582–590 (2002).
- Ng EH, Chan CC, Yeung WS, Ho PC: Comparison of ovarian stromal blood flow between fertile women with normal ovaries and infertile women with polycystic ovary syndrome. Hum. Reprod. 20, 1881–1886 (2005).
- Walker KF, Jayaprakasan K, Raine-Fenning NJ: Ultrasound in benign gynaecology. Obstet. Gynaecol. Reprod. Med. 17, 33–44 (2007).
- Raine-Fenning N, Jayaprakasan K, Deb S: Three-dimensional ultrasonographic characteristics of endometriomata. Ultrasound Obstet. Gynecol. 31, 718–724 (2008).
- Candiani M, Barbieri M, Bottani B et al.: Ovarian recovery after laparoscopic enucleation of ovarian cysts: insights from echographic short-term postsurgical follow-up. J. Minim. Invasive Gynecol. 12, 409–414 (2005).
- Somigliana E, Ragni G, Benedetti F, Borroni R, Vegetti W, Crosignani PG: Does laparoscopic excision of endometriotic ovarian cysts significantly affect ovarian reserve? Insights from IVF cycles. Hum. Reprod. 18, 2450–2453 (2003).
- Demirol A, Guven S, Baykal C, Gurgan T: Effect of endometrioma cystectomy on IVF outcome: a prospective randomized study. Reprod. Biomed. Online 12, 639–643 (2006).
- Somigliana E, Arnoldi M, Benaglia L, Iemmello R, Nicolosi AE, Ragni G: IVF-ICSI outcome in women operated on for bilateral endometriomas. Hum. Reprod. 23, 1526–1530 (2008).
- Garcia-Velasco JA, Somigliana E: Management of endometriomas in women requiring IVF: to touch or not to touch. Hum. Reprod. 24, 496–501 (2009).
- Alborzi S, Ravanbakhsh R, Parsanezhad ME, Alborzi M, Dehbashi S: A comparison of follicular response of ovaries to ovulation induction after laparoscopic ovarian cystectomy or fenestration and coagulation versus normal ovaries in patients with endometrioma. Fertil. Steril. 88, 507–509 (2007).
- National collaborating centre for Women’s and Children’s Health for the National Institute for Clinical Excellence: Fertility: Assessment and Treatment for People with Fertility Problems. RCOG Press, London, UK, 33 (2004). & Guidelines for fertility management.
- Strandell A: Treatment of hydrosalpinx in the patient undergoing assisted reproduction. Curr. Opin. Obstet. Gynecol. 19, 360–365 (2007).
- Nahum GG: Uterine anomalies. How common are they, and what is their distribution among subtypes? J. Reprod. Med. 43, 877–887 (1998).
- Saravelos SH, Cocksedge KA, Li TC: Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: a critical appraisal. Hum. Reprod. Update 14, 415–429 (2008). & Systematic review of prevalence and diagnosis of congenital uterine anomalies.
- Raga F, Bauset C, Remohi J, Bonilla-Musoles F, Simon C, Pellicer A: Reproductive impact of congenital Mullerian anomalies. Human Reprod. 12, 2277–2281 (1997).
- Mazouni C, Girard G, Deter R, Haumonte J-B, Blanc B, Bretelle F: Diagnosis of Mullerian anomalies in adults: evaluation of practice. Fertil. Steril. 89, 219–222 (2008).
- Troiano RN, McCarthy SM: Mullerian duct anomalies: imaging and clinical issues. Radiology 233, 19–34 (2004).
- Shokeir T, Abdelshaheed M: Sonohysterography as a first-line evaluation for uterine abnormalities in women with recurrent failed in vitro fertilization-embryo transfer. Fertil. Steril. 91, 1321–1322 (2009).
- Jurkovic D, Geipel A, Gruboeck K, Jauniaux E, Natucci M, Campbell S: Three-dimensional ultrasound for the assessment of uterine anatomy and detection of congenital anomalies: a comparison with hysterosalpingography and two-dimensional sonography. Ultrasound Obstet. Gynecol. 5, 233–237 (1995).
- Raga F, Bonilla-Musoles F, Blanes J, Osborne NG: Congenital Mullerian anomalies: diagnostic accuracy of three-dimensional ultrasound. Fertil. Steril. 65, 523–528 (1996).
- Church DG, Vancil JM, Vasanawala SS: Magnetic resonance imaging for uterine and vaginal anomalies. Curr. Opin. Obstet. Gynecol. 21, 379–389 (2009).
- Lin PC: Reproductive outcomes in women with uterine anomalies. J. Womens Health (Larchmt) 13, 33–39 (2004).
- Valli E, Vaquero E, Lazzarin N, Caserta D, Marconi D, Zupi E: Hysteroscopic metroplasty improves gestational outcome in women with recurrent spontaneous abortion. J. Am. Assoc. Gynecol. Laparosc. 11, 240–244 (2004).
- Salim R, Jurkovic D: Assessing congenital uterine anomalies: the role of threedimensional ultrasonography. Best Pract. Res. Clin. Obstet. Gynaecol. 18, 29–36 (2004). & Reviews 3D ultrasound diagnosis of uterine anomalies using modified American Fertility Society classification.
- Oliveira FG, Abdelmassih VG, Diamond MP, Dozortsev D, Melo NR, Abdelmassih R: Impact of subserosal and intramural uterine fibroids that do not distort the endometrial cavity on the outcome of in vitro fertilization-intracytoplasmic sperm injection. Fertil. Steril. 81, 582–587 (2004).
- Sunkara SK, Khairy M, El-Toukhy T, Khalaf Y, Coomarasamy A: The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Hum. Reprod. 25, 418–429 (2009).
- Isikoglu M, Berkkanoglu M, Senturk Z, Coetzee K, Ozgur K: Endometrial polyps smaller than 1.5 cm do not affect ICSI outcome. Reprod. Biomed. Online 12, 199–204 (2006).
- Bartkowiak R, Kaminski P, Wielgos M, Bobrowska K: The evaluation of uterine cavity with saline infusion sonohysterography and hysteroscopy in infertile patients. Neuro. Endocrinol. Lett. 27, 523–528 (2006).
- te Velde ER, Pearson PL: The variability of female reproductive ageing. Hum. Reprod. Update 8, 141–154 (2002).
- Broekmans FJ, Faddy MJ, Scheffer G, te Velde ER: Antral follicle counts are related to age at natural fertility loss and age at menopause. Menopause 11, 607–614 (2004).
- de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, van Leeuwen FE: Increased risk of early menopausal transition and natural menopause after poor response at first IVF treatment. Hum. Reprod. 18, 1544–1552 (2003).
- Klinkert ER, Broekmans FJ, Looman CW, Habbema JD, te Velde ER: The antral follicle count is a better marker than basal folliclestimulating hormone for the selection of older patients with acceptable pregnancy prospects after in vitro fertilization. Fertil. Steril. 83, 811–814 (2005).
- Syrop CH, Dawson JD, Husman KJ, Sparks AE, Van Voorhis BJ: Ovarian volume may predict assisted reproductive outcomes better than follicle stimulating hormone concentration on day 3. Hum. Reprod. 14, 1752–1756 (1999).
- Bowen S, Norian J, Santoro N, Pal L: Simple tools for assessment of ovarian reserve (OR): individual ovarian dimensions are reliable predictors of ovarian reserve. Fertil. Steril. 88, 390–395 (2007).
- Elgindy EA, El-Haieg DO, El-Sebaey A: Anti-Mullerian hormone: correlation of early follicular, ovulatory and midluteal levels with ovarian response and cycle outcome in intracytoplasmic sperm injection patients. Fertil. Steril. 89, 1670–1676 (2008).
- McIlveen M, Skull JD, Ledger WL: Evaluation of the utility of multiple endocrine and ultrasound measures of ovarian reserve in the prediction of cycle cancellation in a high-risk IVF population. Hum. Reprod. 22, 778–785 (2007).
- Schild RL, Knobloch C, Dorn C, Fimmers R, van der Ven H, Hansmann M: The role of ovarian volume in an in vitro fertilization programme as assessed by 3D ultrasound. Arch. Gynecol. Obstet. 265, 67–72 (2001).
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB: A systematic review of tests predicting ovarian reserve and IVF outcome. Hum. Reprod. Update 12, 685–718 (2006).
- Jayaprakasan K, Campbell BK, Clewes JS, Johnson IR, Raine-Fenning NJ: Threedimensional ultrasound improves the interobserver reliability of antral follicle counts and facilitates increased clinical work flow. Ultrasound Obstet. Gynecol. 31, 439–444 (2008).
- Scheffer GJ, Broekmans FJ, Looman CW et al.: The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum. Reprod. 18, 700–706 (2003).
- Scheffer GJ, Broekmans FJ, Bancsi LF, Habbema JD, Looman CW, Te Velde ER: Quantitative transvaginal two- and three-dimensional sonography of the ovaries: reproducibility of antral follicle counts. Ultrasound Obstet. Gynecol. 20, 270–275 (2002).
- Jayaprakasan K, Walker KF, Clewes JS, Johnson IR, Raine-Fenning NJ: The interobserver reliability of off-line antral follicle counts made from stored threedimensional ultrasound data: a comparative study of different measurement techniques. Ultrasound Obstet. Gynecol. 29, 335–341 (2007).
- Verhagen TE, Hendriks DJ, Bancsi LF, Mol BW, Broekmans FJ: The accuracy of multivariate models predicting ovarian reserve and pregnancy after in vitro fertilization: a meta-analysis. Hum. Reprod. Update 14, 95–100 (2008).
- Raine-Fenning N, Jayaprakasan K, Clewes J et al.: SonoAVC: a novel method of automatic volume calculation. Ultrasound Obstet. Gynecol. 31, 691–696 (2008). & First original article on validation of automated volume calculation using Sonography-based Automated Volume Count™.
- Deb S, Jayaprakasan K, Campbell BK, Clewes JS, Johnson IR, Raine-Fenning NJ: Intraobserver and interobserver reliability of automated antral follicle counts made using three-dimensional ultrasound and SonoAVC. Ultrasound Obstet. Gynecol. 33, 477–483 (2009).
- Zaidi J, Barber J, Kyei-Mensah A, Bekir J, Campbell S, Tan SL: Relationship of ovarian stromal blood flow at the baseline ultrasound scan to subsequent follicular response in an in vitro fertilization program. Obstet. Gynecol. 88, 779–784 (1996).
- Engmann L, Sladkevicius P, Agrawal R, Bekir JS, Campbell S, Tan SL: Value of ovarian stromal blood flow velocity measurement after pituitary suppression in the prediction of ovarian responsiveness and outcome of in vitro fertilization treatment. Fertil. Steril. 71, 22–29 (1999).
- Kupesic S, Kurjak A, Bjelos D, Vujisic S: Three-dimensional ultrasonographic ovarian measurements and in vitro fertilization outcome are related to age. Fertil. Steril. 79, 190–197 (2003).
- Ng EH, Tang OS, Chan CC, Ho PC: Ovarian stromal blood flow in the prediction of ovarian response during in vitro fertilization treatment. Hum. Reprod. 20, 3147–3151 (2005).
- Jayaprakasan K, Al-Hasie H, Jayaprakasan R et al.: The three-dimensional ultrasonographic ovarian vascularity of women developing poor ovarian response during assisted reproduction treatment and its predictive value. Fertil. Steril. 92(6), 1862–1869 (2008).
- Penzias AS, Emmi AM, Dubey AK, Layman LC, DeCherney AH, Reindollar RH: Ultrasound prediction of follicle volume: is the mean diameter reflective? Fertil. Steril. 62, 1274–1276 (1994).
- Wittmaack FM, Kreger DO, Blasco L, Tureck RW, Mastroianni L Jr, Lessey BA: Effect of follicular size on oocyte retrieval, fertilization, cleavage, and embryo quality in in vitro fertilization cycles: a 6-year data collection. Fertil. Steril. 62, 1205–1210 (1994).
- Nataprawira DS, Harada T, Sekijima A, Mio Y, Terakawa N: Assessment of follicular maturity by follicular diameter and fluid volume in a program of in vitro fertilization and embryo transfer. Asia Oceania J. Obstet. Gynaecol. 18, 225–230 (1992).
- Raine-Fenning N, Jayaprakasan K, Clewes J: Automated follicle tracking facilitates standardization and may improve work flow. Ultrasound Obstet. Gynecol. 30, 1015–1018 (2007).
- Raine-Fenning N, Deb S, Jayaprakasan K, Clewes J, Hopkisson J, Campbell B: Timing of oocyte maturation and egg collection during controlled ovarian stimulation: a randomized controlled trial evaluating manual and automated measurements of follicle diameter. Fertil. Steril. (2009) (Epub ahead of print).
- Abou-Setta AM, Mansour RT, Al-Inany HG, Aboulghar MM, Aboulghar MA, Serour GI: Among women undergoing embryo transfer, is the probability of pregnancy and live birth improved with ultrasound guidance over clinical touch alone? A systemic review and meta-analysis of prospective randomized trials. Fertil. Steril. 88, 333–341 (2007).
- Coroleu B, Barri PN, Carreras O et al.: The influence of the depth of embryo replacement into the uterine cavity on implantation rates after IVF: a controlled, ultrasound-guided study. Hum. Reprod. 17, 341–346 (2002).
- Franco JG Jr, Martins AM, Baruffi RL et al.: Best site for embryo transfer: the upper or lower half of endometrial cavity? Hum. Reprod. 19, 1785–1790 (2004).
- Drakeley AJ, Jorgensen A, Sklavounos J et al.: A randomized controlled clinical trial of 2295 ultrasound-guided embryo transfers. Hum. Reprod. 23, 1101–1106 (2008).
- Kosmas IP, Janssens R, De Munck L et al.: Ultrasound-guided embryo transfer does not offer any benefit in clinical outcome: a randomized controlled trial. Hum. Reprod. 22, 1327–1334 (2007).
- Matorras R, Urquijo E, Mendoza R, Corcostegui B, Exposito A, Rodriguez-Escudero FJ: Ultrasound-guided embryo transfer improves pregnancy rates and increases the frequency of easy transfers. Hum. Reprod. 17, 1762–1766 (2002).
- Sallam HN, Sadek SS: Ultrasound-guided embryo transfer: a meta-analysis of randomized controlled trials. Fertil. Steril. 80, 1042–1046 (2003).
- Sallam HN, Agameya AF, Rahman AF, Ezzeldin F, Sallam AN: Ultrasound measurement of the uterocervical angle before embryo transfer: a prospective controlled study. Hum. Reprod. 17, 1767–1772 (2002).
- Hofmann GE, Warikoo P, Jacobs W: Ultrasound detection of pyometra at the time of embryo transfer after ovum retrieval for in vitro fertilization. Fertil. Steril. 80, 637–638 (2003).
- Senturk LM, Erel CT: Thin endometrium in assisted reproductive technology. Curr. Opin. Obstet. Gynecol. 20, 221–228 (2008).
- Shapiro H, Cowell C, Casper RF: The use of vaginal ultrasound for monitoring endometrial preparation in a donor oocyte program. Fertil. Steril. 59, 1055–1058 (1993).
- Gonen Y, Casper RF: Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF). J. In Vitro Fert. Embryo Transf. 7, 146–152 (1990).
- Richter KS, Bugge KR, Bromer JG, Levy MJ: Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil. Steril. 87, 53–59 (2007).
- Killick SR: Ultrasound and the receptivity of the endometrium. Reprod. Biomed. Online 15, 63–67 (2007).
- Fanchin R, Righini C, Ayoubi JM, Olivennes F, de Ziegler D, Frydman R: New look at endometrial echogenicity: objective computer-assisted measurements predict endometrial receptivity in in vitro fertilization-embryo transfer. Fertil. Steril. 74, 274–281 (2000).
- Bosch E, Valencia I, Escudero E et al.: Premature luteinization during gonadotropinreleasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil. Steril. 80, 1444–1449 (2003).
- Melo MA, Meseguer M, Garrido N, Bosch E, Pellicer A, Remohi J: The significance of premature luteinization in an oocyte-donation programme. Hum. Reprod. 21, 1503–1507 (2006).
- Friedler S, Schenker JG, Herman A, Lewin A: The role of ultrasonography in the evaluation of endometrial receptivity following assisted reproductive treatments: a critical review. Hum. Reprod. Update 2, 323–335 (1996).
- Jinno M, Ozaki T, Iwashita M, Nakamura Y, Kudo A, Hirano H: Measurement of endometrial tissue blood flow: a novel way to assess uterine receptivity for implantation. Fertil. Steril. 76, 1168–1174 (2001).
- Zaidi J, Campbell S, Pittrof R, Tan SL: Endometrial thickness, morphology, vascular penetration and velocimetry in predicting implantation in an in vitro fertilization program. Ultrasound Obstet. Gynecol. 6, 191–198 (1995).
- Kupesic S, Bekavac I, Bjelos D, Kurjak A: Assessment of endometrial receptivity by transvaginal color Doppler and threedimensional power Doppler ultrasonography in patients undergoing in vitro fertilization procedures. J. Ultrasound Med. 20, 125–134 (2001).
- Raine-Fenning NJ, Campbell BK, Kendall NR, Clewes JS, Johnson IR: Endometrial and subendometrial perfusion are impaired in women with unexplained subfertility. Hum. Reprod. 19, 2605–2614 (2004).
- Alcazar JL: Three-dimensional ultrasound assessment of endometrial receptivity: a review. Reprod. Biol. Endocrinol. 4, 56 (2006). & Overview of the current predictors of endometrial receptivity.
- Fanchin R, Righini C, Olivennes F, Taylor S, de Ziegler D, Frydman R: Uterine contractions at the time of embryo transfer alter pregnancy rates after in-vitro fertilization. Hum. Reprod. 13, 1968–1974 (1998).
- Lesny P, Killick SR: The junctional zone of the uterus and its contractions. BJOG 111, 1182–1189 (2004).