Editorial - Imaging in Medicine (2011) Volume 3, Issue 2
Ultrasound evaluation of hepatic vein morphology: a promising simple diagnostic tool?
Robert N Gibson*Department of Radiology, University of Melbourne, Royal Melbourne Hospital, Victoria 3050, Australia
- Corresponding Author:
- Robert N Gibson
Department of Radiology
University of Melbourne
Royal Melbourne Hospital, Victoria 3050, Australia
E-mail: r.gibson@unimelb.edu.au
Abstract
Keywords
cirrhosis ▪ fibrosis ▪ liver
Considerable advances have been made in the noninvasive assessment of diffuse liver disease. Much of the focus has been on attempts at staging fibrosis without resorting to liver biopsy with its accompanying morbidity. Quantifying the extent or stage of fibrosis is important prognostically, in deciding on treatment, assessing response to treatment and in deciding when to commence surveillance for hepatocellular carcinoma.
Much of the recent interest and research activity has been in the use of techniques using imaging- related technology. The main area has been in measurement of liver elasticity using ultrasound [1] or MRI [2]. As liver fibrosis increases the liver becomes less elastic and this change in elasticity can be measured with some degree of accuracy and reproducibility. A second and, to date, less convenient and robust approach, has been the measurement of transit time of microbubble ultrasound contrast agents through the liver [3]. Microbubble transit time tends to decrease with development of more severe fibrosis and cirrhosis, most likely as a result of intrahepatic shunting.
“...the accurate noninvasive diagnosis of cirrhosis and staging of fibrosis is still something of a ‘holy grail’.”
These techniques have reached variable maturity and, in some cases, most notably transient elastography, have been quite widely adopted. However, they do have limitations in terms of technical failure in some patients and less than ideal reproducible accuracy. Liver stiffness measurements have been shown to be influenced by factors other than fibrosis; for example, the degree of acute inflammatory activity [4]. This can result in liver stiffness measurements measurements falsely predicting cirrhosis. Therefore, the accurate noninvasive diagnosis of cirrhosis and staging of fibrosis is still something of a ‘holy grail’.
Whilst attention has focused on differentiating between stages of fibrosis, one issue that has been sidelined to some extent is the simpler question of whether or not the patient has cirrhosis, the most advanced grade of fibrosis. Recent work has shown that detailed examination of the hepatic vein walls by conventional ultrasound appears to be a relatively accurate technique for diagnosing or excluding cirrhosis [5].
The attraction of the technique is that it can be performed using conventional ultrasound equipment used for imaging, and does not require additional machine capability for measuring elasticity. Therefore, it is already widely available and relatively inexpensive technology.
The idea of evaluation of hepatic vein morphology by ultrasound is a simple one, in that the nodularity of the liver intrinsic to cirrhosis is best appreciated at liver parenchymal boundaries. Historically, for ultrasound this has been the external liver capsule, and the sensitivity for diagnosis of cirrhosis using this technique has been relatively low and highly variable with a reported range of 54 to 88% [6–8]. The hepatic veins are a potentially more attractive parenchymal boundary for ultrasound, as they provide a natural interface between solid tissue (the liver parenchyma) and liquid (hepatic venous blood), two tissues with very different acoustic impedance and echogenicity (as occurs at the liver surface surrounded by ascites). The result is an interface between tissues high in ultrasound contrast and one that creates a strong specular reflection that is normally smooth. Furthermore, unlike portal veins, which have significant surrounding connective tissue in the portal tracts, the hepatic vein wall is relatively thin so any nodularity of the liver will be more readily appreciated at this boundary.
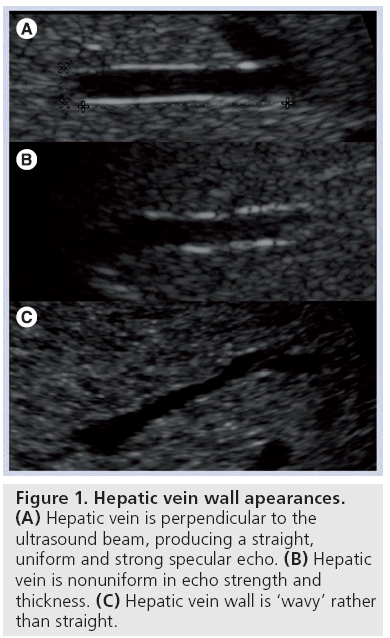
Vessal et al. evaluated the straightness, as well as the echo uniformity, of the hepatic vein wall in normal patients and patients with cirrhosis [5]. The normal hepatic vein wall, when imaged with the ultrasound beam perpendicular to the vein, is a thin bright smooth echogenic line (Figure 1A). Abnormal findings are loss of continuity and uniformity of the echo brightness and thickness (Figure 1B), and loss of smoothness (i.e., the line becomes wavy; Figure 1A).
The combination of lack of straightness and nonuniformity of the vein wall yielded a sensitivity of 88% (95% CI: 73–97%) and specificity of 95% (95% CI: 84–99%) for the diagnosis of cirrhosis with moderately good to very good intraobserver and interobserver agreement. Slightly better results, particularly for the vein wall uniformity parameter, were achieved with compound scanning than noncompound scanning.
Conversely, the finding of a uniform and straight vein wall, perhaps unsurprisingly, had a very high negative predictive value for cirrhosis.
This approach is substantially better than most results relying on ultrasound display of liver surface nodularity with sensitivity of 54–88% and specificity of 64–95% for the diagnosis of cirrhosis [6–8]. Vessal et al. did not make a direct comparison in their cohort between hepatic vein wall morphology and liver surface nodularity (that question is the subject of a current study at the same center) [5].
“...the evaluation of hepatic vein wall morphology appears to offer the best method for identifying cirrhosis using conventional grayscale ultrasound imaging.”
The technique described by Vessal et al. made use of the right hepatic vein, close to its junction with the inferior vena cava. The group has since modified the technique to examine a more peripheral vein in segment 5 or 6 using an intercostal oblique coronal plane to allow a more superficial hepatic vein to be examined with higher resolution. The technique relies on having a perpendicular interface with the vein to appreciate the more subtle changes in the appearance of the hepatic vein wall. A vein segment length of at least 15 mm and diameter of at least 3 mm is chosen, albeit somewhat empirically.
The explanation of the wall changes is interesting to consider. The loss of straightness (wavy appearance) is readily understandable in terms of the contour changes in the hepatic vein wall due to nodule formation in cirrhosis. The nonuniformity of the wall echogenicity also results, at least in part, from these contour changes. It is probable, however, that nonuniformity, or ‘breaks’, in the vein wall also result from changes to the ultrasound beamintensity profile as it passes through bands or zones of fibrosis that develop before cirrhosis develops. If this is the case, then nonuniformity of the vein wall might be expected, at least in the more advanced stages of precirrhotic fibrosis. This is the subject of further study in our department but, to date, it has been observed in cases of F3 fibrosis in hepatitis C [Gibson RN, Unpublished Data] [9].
It is possible, therefore, that detailed study of the hepatic vein walls, in the manner outlined above, will allow recognition of both stage F3 and F4 liver fibrosis, the latter equating to cirrhosis, as well as being useful in differentiating between the two stages.
This requires more study, but, on the basis of evidence to date, the evaluation of hepatic vein wall morphology appears to offer the best method for identifying cirrhosis using conventional grayscale ultrasound imaging, which has the appeal of being cheap and widely available. It may also prove of value in the recognition of precirrhotic fibrosis.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Sandrin L, Fourquet B, Hasquenoph J-M et al.: Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 29, 1705–1713 (2003).
- Taouli B, Ehman RL, Reeder SB: Advanced MRI methods for assessment of chronic liver disease. Am. J. Roentgenol. 193, 14–27 (2009).
- Lim AKP, Taylor-Robinson SD, Patel N et al.: Hepatic vein transit times using a microbubble agent can predict disease severity non-invasively in patients with hepatitis C. Gut 54, 128–133 (2005).
- Taylor-Robinson SD, Cobbold JFL, Thomas HC: Liver stiffness measurements in acute hepatitis B: Implications for clinical practice. Eur. J. Gastroenterol. Hepatol. 22, 133–134 (2010).
- Vessal S, Naidoo S, Hodson J, Stella DL, Gibson RN: Hepatic vein morphology: a new sonographic diagnostic parameter in the investigation of cirrhosis? J. Ultrasound Med. 28, 1219–1227 (2009).
- Filly RA, Reddy SG, Nalbandian AB, Lu Y, Callen PW: Sonographic evaluation of liver nodularity: inspection of deep versus superficial surfaces of the liver. J. Clin. Ultrasound 30, 399–407 (2002).
- Di Lelio A, Cestari C, Lomazzi A, Beretta L: Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 172, 389–392 (1989).
- Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D: Severe liver fibrosis or cirrhosis: accuracy of US for detection – analysis of 300 cases. Radiology 227, 89–94 (2003).
- Bedossa P, Bioulac-Sage P, Callard P et al.: Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 20, 15–20 (1994).