Research Article - Pharmaceutical Bioprocessing (2018) Volume 6, Issue 2
Understanding the role of resveratrol in major neurological and lifestyle diseases: an insight into molecular mechanisms and druggability
Amarendranath Choudhury1,2*, Rudrarup Bhattacharjee2, Dattatreya Adapa3, Indrajeet Chakraborty4, Tuhin Subhra Banerjee5, Dhilleshwara Rao Vana6
1Independent Researcher, Kondapur, Hyderabad-500084, India
2Alumnus, Department of Life Science and Bioinformatics, Assam University, Silchar-788011, India
3Department of Microbiology, Food Science and Technology, GITAM Institute of Sciences, GITAM University, Visakhapatnam- 530045, Andhra Pradesh, India
4Department of Life Science and Bioinformatics, Karunya University, Tamil Nadu-641114, India
5Assitant Teacher of Life Sciences (H/PG), Satpalsa High School, Satpalsa, Birbhum, West Bengal, India
6Center for Excellence in Genomics, MKU, Tamil Nadu, India
- *Corresponding Author:
- Amarendranath Choudhury
Independent Researcher, Kondapur, Hyderabad-500084, India
E-mail: anc.au@hotmail.com
Abstract
Progressive research of last five decades has gathered encourageable data on many edible plant products which showed protective and ameliorative efficacy against various disease profiles. Several medicinal formulations also have targeted such plant products as an alternative drug-source for the treatment of cardiovascular complications, ageing, cancer, arthritis, type 2 diabetes, and neurodegenerative disorders. Among several phytocomponents, Resveratrol (RES) is notable for robust functional contributions and ameliorative properties. RES is considered as one of the most potent naturally occurring polyphenol to fight against oxidative stress, inflammation, and apoptosis. RES is abundantly found in grapes, red wine, peanuts, and some berries. Preclinical experimental studies carried out in this regard, have shown that RES possess numerous biological activities, with possible therapeutic effects in the prevention and treatment of many commonly occurring diseases. In oral administration spectrum, RES was found to be rapidly absorbed by human body; but, owing to high rate of metabolism, exhibiting relatively low bioavailability. In short-term supplementation, it significantly improves the metabolic disorders in patients with type 2 diabetes and other glucose and lipid-induced complications. Experimental studies suggested that, RES exerts beneficial contribution in the prevention of neurodegenerative disorders and cancer; however, the exact mechanisms of action are yet to demonstrate. Moreover, clinical trials are currently very limited and therefore, more research is the need of time. In the present review we have accumulated most of the beneficial contributions of RES and highlighted the possible therapeutic interventions against major disease profiles.
Keywords
polyphenol, resveratrol, neuroprotection, cardiovascular diseases, cognitive impairment, anti-cancer
Introduction
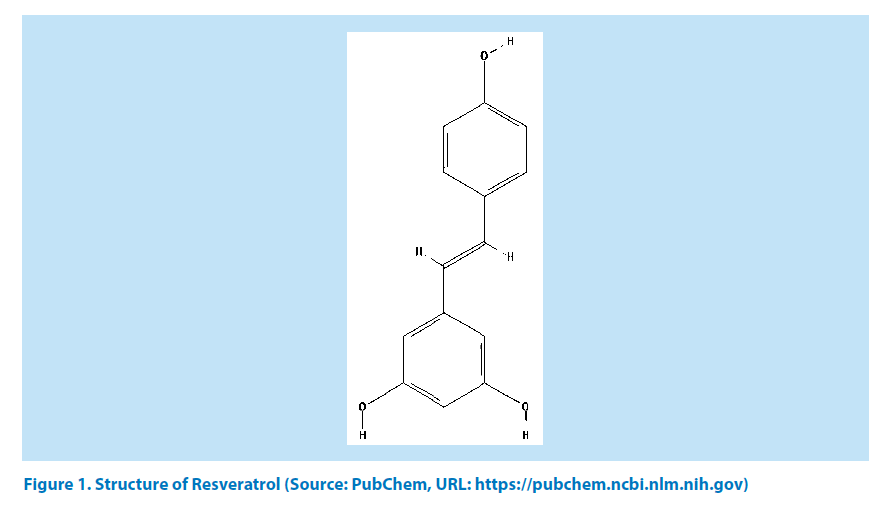
The contribution of natural polyphenols in medicinal chemistry is noteworthy [1-3]. By definition, natural polyphenols are naturally occurring phytochemical compounds with phenolic backbone and abundantly present in secondary metabolites of plant [4]. Besides serving as the most abundant antioxidants for plant, they are also enriching our diet and maintain the same activity in our body. Over the past few years, nutritionists have studied the therapeutic effects of such polyphenols for better understanding about their efficacy [5-8]. Among countless polyphenolic compound, Resveratrol (RES; IUPAC: 5-[(E)-2-(4-hydroxyphenyl) ethenyl] benzene-1,3-diol) is the point of discussion in the present study [9]. Research from last few decades has documented several promising contributions of RES and the same has helped to gain enormous attention from different research community, worldwide. Chemically, RES is a polyphenol phytoalexin stilbene compound; found to exist naturally in grapes and consequently present in red wine, which having strong antioxidant properties (Figure 1) [9,10].
Figure 1. Structure of Resveratrol (Source: PubChem, URL: https://pubchem.ncbi.nlm.nih.gov)
Naturally, RES is found in high quantity in grapes and berries of Vaccinium species, which includes blueberries, bilberries, and cranberries. It is also available in peanut, cocoa, red wine and various other herbs in minor quantity [11-13]. Reports highlighted that; RES has robust biological contributions which includes antioxidant, anti-carcinogenic, anti-inflammatory and chemo-preventive properties [14-18]. Owing to its fat soluble nature, it is observed to be present in both -cis and -trans conformations. During chemical synthesis, RES originates from p-coumaroyl CoA and malonyl CoA, both of which are found to be present in above mentioned plants [19,20].
RES synthase-an enzyme abundantly available in various herbal sources, is essential for RES biosynthesis [21]. High bioavailability of RES synthase is associated with infection, injury, UV-irradiation, and other phyto-stress. The anti-fungal activity of RES enhances disease resistance among plants due to its phytoalexin role [22,23]. Michio Takaoka first isolated RES from a poisonous medicinal plant named Veratrum album and documented in the journal of Imperial University Hokkaido School of Science, 1939. The word ‘Resveratrol’ is derived from the fact that it is a resorcinol derivative found in Veratrum species [24].
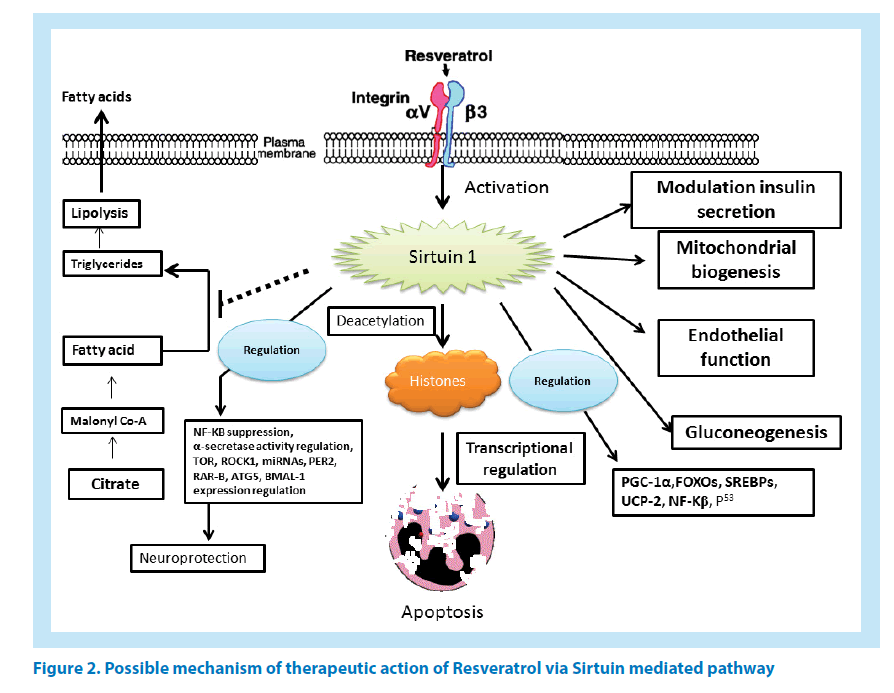
RES attenuates a number of pathogenic biological processes. RES promotes neuroprotection by regulating Aβ-induced apoptosis, and cytotoxicity. In in vivo studies it showed protection from Aβ- induced apoptosis in PC12 cell-line, which further highlighted its efficacy in the regulation of silent information regulator 1. RES also effective in the reduction of intracellular accumulation of reactive oxygen intermediate (ROI) in PC12 cells [25]. The RES (SIRT1)-Rho-associated kinase 1 (ROCK1) signalling pathway also showed similar outcomes [26]. A Mechanistic insight of Sirtuin mediated resveratrol action has been depicted in Figure 2.
Metabolism of resveratrol
RES metabolism determines the effectiveness of RES in biological system. Unfortunately, due to reactive nature of RES, less bio-availability and rapid excretion rate have been reported [27]. Study showed that, oral administration of RES could provide high concentration of RES and related secondary metabolites in peripheral circulation, which existed for ~30 minutes after administration [28]. Depending upon the source of intake, concentration of RES could vary from 416 to 471 μg/L. Intriguingly, Grapes have lower bioavailability of cis-RES as compared to trans-RES owing to presence of glycosides [27]. Studies involving red wine consumption bring out that levels of RES are high in the event of consumption with a meal and when it consumed in empty stomach condition [29,30].
Resveratrol as dietary supplements
It has been reported that, RES is present in several plant and herbs but, availability and quantity of RES depends on several factors. The quantity of RES varies depending upon two crucial factors like geographical origin of cultivation, and exposure to fungal infection [11,31-33]. Also there are varieties in RES availability in different parts of plants. In case of Grape, RES mostly resides in its skin [34,35]. RES content of red wines is proportional to the quantity of grape skin was used to prepare the wine [22]. The predominant form of RES available in grapes and grape juice is trans-resveratrol-3-O-β-glucoside (transpiceid). However, the RES found in wines is actually RES aglycones, resulting from the chemical reaction of sugar cleavage which occurs during fermentation [31].
Presence of significant quantities of cis-RES is believed to be produced during fermentation or released from viniferins (RES polymers) [36]. Even though, red wine has relatively high concentration of biologically available RES, co-existence of other polyphenols at considerably higher concentrations makes the experimentation process complicated [30].
Concentration of RES in peanut is comparable with that in grapes. Sprouting of peanut (subject to cultivar type) influences the levels of RES available in peanut [21]. Evidence advocate that RES concentration is limited to 2.3 - 4.5 μg/g before sprouting which afterwards elevates to a range of 11.7 - 25.7 μg/g [37]. Food products like cocoa powder, baking chocolate, and dark chocolate exhibit low levels of RES in the blood following normal consumption [37]. Red wines are found to contain 0.3 to 2.1 mg/l of trans-RES as compared to white wine that has nearly 0.1 mg/L of trans-resveratrol (both cis- & trans- conformations). In another prospective review, trans-RES concentrations averaged at around 1.9 ± 1.7 mg /L (8.2 ± 7.5 μM) with an undetectable level ranging from 14.3 mg/l (~62.7 μM) [38].
Biological efficacy of resveratrol
Biological efficacy and function of RES has less been studied in clinical paradigm. Past investigations have mainly been conducted in-vitro. Reason behind such fact is that, preclinical experiments are possible only at minimal RES concentration unlikely to be achieved in animal subjects through oral administration [39,40]. Few of them noted biological activities exhibited by RES include direct antioxidant activity, which includes estrogenic and anti-estrogenic activities; biological activities related to cancer prevention, cardiovascular disease prevention, neurodegenerative disease prevention and all related treatments [14,41-43]. RES proves particularly useful in the treatment of type 2 diabetes mellitus by hindering with functioning of cytocrome P450 enzyme avoiding all forms of exposure to carcinogens reducing chances of cancer cell growth [44]. Most significant activities of RES have been elaborated in the sections below:
Efficacy of Resveratrol in cardiovascular diseases
Existing knowledge relating to cardiovascular disease risk is associated with moderate levels of alcohol consumption [45]. In this regard, the ‘French Paradox’ is notable, where it was observed that onset of coronary heart disease was comparatively low in France population despite dietary consumption of saturated fats, cigarette smoking, and other similar habits [46]. This led to a general belief that regular red wine consumption could be helpful in protection from cardiovascular disease [47]. Red wine contains RES in variable levels along with high flavonoids concentrations such as procyanidins, which has been showed profound anti-oxidant, anti-inflammatory, anti-atherogenic activities [48].
Epidemiological studies carried out in this regards are also supported such contention. Despite the fact that some earlier studies showed lower risk of cardiovascular disease among wine drinkers when compared to beer/liquor drinkers [49-51], while in case of other reports, no difference was noted [52-54]. Differences in lifestyle and health of people, preferring wine over other liquor may explain some of therapeutic benefits as observed experimentally [55]. Effects of aging are often observed as cardiovascular risk; directly expressed as an inhibition of vascular cell adhesion molecule expression, vascular smooth muscle cell proliferation, platelet activation and aggregation, besides stimulation of endothelial nitric oxide synthase activity, which may be observed even when associated risk factor remain unaffected viz. hypercholesterolemia, diabetes, hypertension, etc. [26,56-59].
Major characteristics of cardiovascular ageing are explained as reduced endothelial functioning together with progressively deteriorating myocardial function [60]. Contemporary research supports the previously mentioned ‘French paradox’ elaborating on the benefits of RES at controlling cardiovascular morbidity [47]. Red wine consumption has been reported to lower bad cholesterol levels, which concurrently reducing blood vessel damages and preventing blood clotting [61].
Blood platelets have fundamental role in atherogenic mechanism, for which aspirin is the most commonly used treatment [62]. Nevertheless, aspirin administration often becomes unsuccessful due to several side-effects [63]. Under such circumstances, RES was found useful in suppressing platelet aggregation consequently offering protection against coronary heart disease [64]. Asprin like effects of RES are attributed to Cyclooxygenase-1 inhibition, which not only makes it a potential substitute for the prevention and treatment of cardiovascular diseases, but also proving particularly useful among subjects facing aspirin resistance [65].
Resveratrol as an antioxidant agent
Experimentation with in vitro setup, RES effectively neutralizes free radicals and oxidants in addition to inhibiting low-density lipoprotein (LDL) oxidation [66,67]. It is also known to induce production of antioxidant enzymes like superoxide dismutase (SOD), thioredoxin, glutathione peroxidase-1, heme oxygenase-1, etc. apart from catalysing, and/or inhibit reactive oxygen species (ROS) production by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) [68-70].
However, clinical evidence concerning the antioxidant activity of RES is not satisfactory [39,71,72]. Following oral intake of RES, circulating and intracellular levels of RES are observed to be much lower as compared to other important antioxidants, such as vitamin C, uric acid, vitamin E, and glutathione [73,74]. Furthermore, antioxidant activity of RES metabolites is also lower than that of RES [75,76].
RES shows effective neutralization of many free radicals, low density lipoprotein, and anti-oxidants. In spite of lower concentration as compared to other anti-oxidants like Vitamin-C, Vitamin-E, etc. anti-oxidant activity of RES is experimentally determined to be superior [77]. Primarily working in upstream reactions, using inactive copper as a catalyst at higher concentrations; it causes vasodilation in conduit arteries and microcirculation along with elicit dilatation of the internal mammary artery in human [66].
In clinical studies, RES exhibit improved vasodilation upon being evoked by endothelium-dependent agents which are beneficial in relaxing the aged arteries through restoration of NO bioavailability [78,79]. Conversely, acute administration of RES remains ineffective due to reduced O2 production in smooth muscle and endothelial cells located in the arteries leading to incomplete and ineffective RES activity [80].
Resveratrol as an anti-inflammatory agent
In murine models, repetitive enteral or intraperitoneal administration of RES at high dosages has shown protective effects in the small intestine against acute ischemia/ reperfusion damages [81]. Additionally, decrease in endothelial VCAM and ICAM-1, expression along with attenuating monocyte adhesiveness to the endothelium, and regulation of expression of inflammatory mediators namely such as cytokines, TNFα, IL-1β, IL-6 and adhesion molecules, are also anti-inflammatory contribution of RES [82]. Herein, NF-kB activation is linked to up-regulation of pro-inflammatory gene products like chemokines, cytokines, and cell adhesion molecules. RES not only activates NF-kB and establishes a predisposition of arteries towards atherosclerosis, but also regulates the functioning of Activator protein-1(AP-1) [83]. AP-1 is another pro-inflammatory transcription factor has been shown to be active under both NF-kB activation and oxidative stress environment [84]. Considering the strong inhibition of NF-kB and related factor, it is confirmed that RES is an important entity to fight against inflammation. Likewise, vascular dysfunctions such as endothelial dysfunction, pro-atherogenic activities, and vascular oxidative stress enhancement are found to be up-regulated by iNOS production. RES acts as an excellent anti-iNOS regulator providing relief in these cases [85].
Resveratrol as an anti-aging agent
Current knowledge on ageing process reveals that therapeutic benefits of different herbal component are effective to reduce the progressive growth of aging [5-8,86- 91]. However, the protective effect of RES in this regards has been observed through Sirtuin-1 (SIRT1) activation and functional regulation [92,93]. SIRT1 affects ageing process and disease progression by accelerating mitochondrial functioning and thereby increasing ATP production [94]. Genomic exploration of the underlying mechanism links SIRT1 production with IA phosphoinositide 3-kinase (PI3K) gene, with the help of which it provides anti-ageing benefits [94]. Anti-ageing effects of RES have already been confirmed in eukaryotes viz. S. Cerevisiae, C. Elegans, and D. Melanogaster [95] wherein, it serves as a calorie/energy restriction mimetic agent [96]. Predominantly, RES administration has showed increased longevity in C. Elegans regulating SIRT1 (NAD-dependent deacetylase sirtuin-1), a protein encoded by the SIRT1 gene. However, regulation of SIRT1 is still not comprehensively understood in higher animals [97].
Resveratrol in the therapeutic intervention for cancer
RES has shown potent anti-proliferation activity on quite a few human cancer cell lines, some of which are breast, prostate, stomach, colon, pancreatic, and thyroid cancer [75]. However, cigarette smoking related lung carcinoma is resistant to oral RES administration [98]. Some findings showed that RES when used ~1 mg/kg body weight orally has a reductive effect on esophageal tumors in rats treated with a carcinogen [99]. It has been reported that, RES functions as a multi-preventive agent against several cancer types by virtue of its quick absorption and metabolism [100]. When administered orally, it quickly gets metabolized and promotes anti-inflammatory, anti-angiogenesis and pro-apoptotic stimulus in many test subjects with a variety of cancer types ranging from skin, breast, lung and so on [100,101]. Jang et al. witnessed the capability of RES as a inducing agent for phase II drug-metabolizing enzymes, thereby preventing related mechanisms associated with cancer progression [102].
RES is also reported to inhibit cyclooxygenase (COX) and hydroperoxidase enzymes showing anti-growth activity against cancerous cells [100,103]. Anti-tumour progression activity is carried out by inducing promyelocytic leukemia cell differentiation factor [103]. In a study involving breast cancer model, it was seen that dose dependent RES administration inhibited estrogen receptor positive MCF-7 cells. RES acted as an antagonist to the 17-b-estradiol (E2), thereby inhibiting its growth promoting functionality which ultimately prevented the progression of breast cancer [104]. RES has shown anti-cancer activity by suppressing the mammalian target of rapamycin (mTOR), which is a key regulator of cell proliferation [105]. Wu et al. identified that multiple components of the phosphatidylinositol 3- kinase(PI3K)/ Akt and mTOR signalling pathways are targeted by RES, which includes PI3K, Akt, PTEN, and DEPTOR [106,107]. Alteration of these pathways effectively regulate cancer progression henceforth, imparting potent anti-cancer characteristics [101]. RES showed inhibitory effect on malignant transformation of rat epithelia, which was experimentally induced by N-nitrosomethylbenzylamine (NMBA) [108].
In another study with Min mice, RES showed inhibitory effect on gastro-intestinal carcinoma [109]. In a study with ovarian cancer cell lines, RES was found to promote apoptosis in malignant cells primarily via autophagocytosis (autophagy or type II programmed cell death). In this case, RES action was not found to be dependent on caspase function or Bcl-2/Bcl-xL expression, rather exhibited typical morphological features of authophagic death upon microscopic examination of dying cells [110]. In a mouse breast cancer model, administration of RES resulted in significant decrease of tumour growth and progression [110]. In experimental nude mice with ER-α negative and ER-β positive MDA-MB-231 tumours, RES treatment increased the apoptotic index and mediated a critical cell death phenomenon [111]. In a study with yeast, it has been found that RES strongly activates yeast SIR2, the homolouge of mammalian SIRT-1, which facilitates increase in the life span of Saccaromyces cerevisiae [112]. Such activity of RES to enhance life was also confirmed in studies with Caenorhabditis elegans and Drosophila melanogaster [113].
More than hundreds of studies have suggested that RES has potent activity against prostate cancer [114]. At very low concentrations, RES was able to induce growth in receptor negative breast cancer cell lines, behaving in a unexplained manner [114]. Being a dietary antioxidant and antitumor substance, RES has a critical role in mediating autophagy as well as apoptosis [115]. Signorelli et al. used Dihydroceramide-an immediate precursor of the apoptotic mediator ceramide in the de novo sphingolipid synthesis pathway, for studying gastric cancer cells [116]. In this study, it was observed that administration of RES induced autophagy in HGC-27 cells and showed no signs of apoptosis. The underlying mechanism of such activity could be due to inhibition of the enzyme dihydroceramide desaturase which resulted in increased dihydroceramides [116]. With growing cases of liver cancer, dietary intake of RES showed promising results against hepatocellular carcinoma [117]. It is observed that anti- HCC actions of RES are mediated through the inhibition of abnormal cell proliferation and apoptosis by cell cycle regulation. Besides, it is known experimentally that RES inhibits the growth of HCC cells and prevents hepatocarcinogenesis by alleviating oxidative stress [100,117].
Resveratrol and apoptosis
Adding to the anti-cancer benefits of RES, apoptosis modulatory function is one of the major attributes in this section. Periodically, experimental studies have discovered many significant pathways through which RES modulates apoptosis in malignant cells [118,119]. A study reported for the first time that RES induced apoptosis is mediated via transactivation of p53 tumor suppressor gene in a dose dependent manner [118]. With advancement of analytical techniques and increasing interest on RES anti-cancer activity, several studies on various trial groups affected with cancer showed interesting results of RES regulating apoptosis in cancer cells [120,121]. Shih et al. reported that RES modulates a Ras-MAPK kinase pathway along with MAPK signal transduction which in turn increases p53 expression and consequent phosphorylation causing p53 dependent apoptosis in the study subjects [121].
One report suggested that RES induces the clustering of Fas and then modulates its redistribution in areas which are rich in cholesterol and sphingolipids in SW480 cells. This clustering also includes FADD and procaspase-8 [122,123]. This kind of redistribution has shown to induce deathinducing signaling complex (DISC) formation, which in turn regulates apoptosis in the concerned study cells [124]. Another study suggested that RES induces apoptosis in melanoma cells by modulating the signalling via ERK1/2 phosphorylation in A375 cell lines [119]. Another study suggested that RES act via multi-pathway modulator to bring about its anti-cancer effect [41]. The molecular mechanisms suggested, for this involves signalling pathways related to extracellular growth factors and receptor tyrosine kinases [125]. As a result, formation of multiprotein complexes and cell metabolism, cell proliferation and genome instability, cytoplasmic tyrosine kinase signaling (cytokine, integrin), signal transduction by the transforming growth factor-β superfamily and immune surveillance and hormone signalling was observed [126].
In a recent study involving breast cancer, the role of RES in inducing apoptosis has been highlighted in the context of miRNA. RES modulates certain miRNA which then regulates key apoptotic and cell cycle related genes like Bcl-2, X-linked inhibitor of apoptosis protein and CDK [127]. Such modulation of miRNAs by RES confirmed its role in inducing cell death via apoptosis in MCF-7 cancer cell lines (Figure 2) [128].
Neuroprotective effects of Resveratrol
RES exhibits effective therapeutic competence in case of different neurological disorders such as Parkinson’s disease (PD), Alzheimer’s disease (AD), etc [129,130]. In experimental animal studies, it has been found that RES showed anti-plaque formation activity in brains of animal subjects. Upon oral administration of RES in humans, it showed reduction in β-amyloid plaque levels which is regarded as an age associated change in the brain [131]. In the subsequent sections, detailed information regarding the protective effects of RES in brain pathology is explained.
Effects of resveratrol in amyloid pathology
Accumulation of pathological products in different regions of the brain cause severe damage to the synapse structure causing impairment of synaptic transmission [132]. Clinical symptoms of AD appear due to deposition of Tau proteins in the brain regions which ultimately results in loss of neuronal functioning [133]. It is important to understand that Aβ production and clearance controls the homeostatic balance in the brain [134]. Meanwhile, β-APP cleaving enzyme (BACE) and β-secretase are responsible for Aβ production through the sequential cleavage of amyloid precursor protein (APP) [135]. Experimental studies reported that receptor-mediated endocytosis along with autophagic pathway were the primary pathways involved in Aβ clearance and degradation [136]. Enzymes such as Aβ-degrading enzymes (ADEs) cleave the Aβ into smaller fragments and in doing so, regulates Aβ metabolism and brain homeostasis through a number of molecular pathways [137]. Transport proteins for instance P-glycoprotein located in astrocytes and abluminal side of the cerebral endothelium transports Aβ to and from brain to blood [138]. This existing knowledge has motivated researchers to search for phytochemicals which directly interact and modify Aβ equilibrium [1]. The aim is to target intermediary steps in Aβ production and clearance mechanism.
In AD pathogenesis, metabolic pathway of Aβ metabolism plays a vital role. Especially, in case of late onset of AD, it has been studied extensively and considered the new lead for the development of therapeutics [139,140]. It was also observed here that Aβ clearance occurs through a number of pathways such as activation of Amyloid-degrading enzymes (ADEs), microglial phagocytic uptake, circulation after crossing BBB levels, and through control of Aβ aggregation [141]. The enzymatic breakdown of proteins in the regions of brain also known as ‘degradation clearance’ entails both extracellular and intracellular degradation. The extracellular degradation of ISF proteins ensues primarily with the help of proteases which are expressed and secreted by astrocytes and other neuronal cells such as phagocytic microglia etc. [142,143]. It has been experimentally determined that degradation of interstitial fluid (ISF) not only takes place in the brain region, rather it may also be absorbed into the astrocyte and glial cells from the surrounding extracellular matrix [144,145]. Likewise, intracellular degradation of proteins takes place through the ubiquitin-proteasome pathway, the autophagy-lysosome pathway, and the endosome-lysosome pathway [146].
At the molecular level, AD induced pathogenesis requires cleavage of extracellular APP by a secretase called BACE1, mainly located in neurons. Tamagno et al. reported that administration of 4-hydroxynonenal (HNE)- an oxidative stress marker, is responsible for increased BACE1 expression which results into uncontrolled APP processing and cytotoxicity via amiloidogenesis [147]. The mechanism underlying such pathogenic action solely depends on accumulation of amyloid beta (Aβ) peptide [147]. Rationale of such realization has attracted researchers to draw therapeutic paradigm taking BACE inhibitors to reduce amiloidogenesis [148]. This idea has presented encouraging results which are useful in the therapeutic treatment of AD pathogenesis [149]. Literature evidences and experimental validations using fluorescence resonance energy transfer (FRET) assay in this context advocates the positive effects of RES and its oligomers as inhibitors of BACE activity in a dose dependent manner [150]. Conformational based activity studies on RES and its derivatives revealed that RES has the ability to alter the functionality of BACE1 depending on the electron affinity of the functional groups. These findings were validated using time-resolved fluorescence (TRF) assay [151] and it was confirmed that RES can effectively inhibit the function of BACE1 [152]. Clinical trials on some of the potent BACE1 inhibitors (LY2886721, E2609, and AZD3293) have failed to yield desired outcome. Reason behind this paradox remains unclear till date nevertheless, it is anticipated that these contradictions may have arisen owing to irregularity in terms of methods and materials used while performing the experiment [153]. Thus, calling for a comprehensive revaluation of RES activity both in-vitro and in-vivo is the urgent need of time [154]. Considering these facts, it is obvious that future therapeutics with regard to neuroprotection shall necessary include RES and other active phytochemicals [88,91,155-158] obtained from herbal sources. This would not only selectively target secretase activity but also works effectively without any side-effects.
In secretase enzymes, the sub-class β-secretase is classified under the family of intramembranous aspartyl proteases which are composed of four subunits, viz. presenilin (PSEN), anterior pharynx defective-1, nicastrin, and presenilin enhancer-2 [159]. Upon proper conjugation, these sub-units impart functional characteristics to the enzyme besides regulating its catalytic activity. It has been noted that even minor changes to the secretase sub-units greatly affects its catalytic activity [160]. In mouse models, RES administration showed notable rise in PSEN1 expression, helping as a SIRT1- specific DNA targets. Nevertheless, clarity on secretase activity, suppression of Aβ by SIRT1 remains unknown [161]. Experimental studies using N2a cells also known as Neuro2a neuroblastoma cells have ascertained the inhibitory activity of RES and its analogues against secretase activity [162]. Results of which can be observed as a decrease in levels of Aβ; however, not causing any cell death in the process. Nevertheless, studies contradicting these outcomes exist as well. Studies by Marambaud associates observed no vital effect of RES on Aβ-producing enzymes β- and γ-secretases. However, it was reported in their study that RES promotes intracellular degradation of Aβ through a proteasome mediated pathway [163].
The plasminogen activator system is an enzymatic cascade involved in the control of fibrin degradation, matrix turnover and cell invasion. It is believed to be associated with neuronal plasticity and long-term potentiation (LTP) in the brain [164]. Experimental findings, in mouse models attribute Aß clearance through tPA-plasmin system [165]. These modulations serve useful in containing Aß-induced neurotoxicity. Observing this, we may comfortably conclude that late onset of AD symptoms may well be related to plasmin system activity [166]. Likewise, AD susceptibility is attributed to u-PA gene polymorphism. In-vitro studies involving human umbilical vein endothelial cells (HUVECs), mediates plasmin activation alongside plasminogen endo-proteolysis [167]. It was reported by several groups that administration of RES enhances the expression of plasminogen activators t-PA and u-PA which further leads to reduction in amiloidogenesis through plasminogen endoproteolysis and plasmin activation [168,169].
Resveratrol and blood-brain barrier
The Blood-Brain Barrier (BBB) protects the brain and spinal cord from damages induced by foreign agents, hormones, and neurotransmitters [170,171]. BBB is responsible for maintaining a uniform environment in regions of the brain and spinal cord. Existing evidences relate clearance of toxic Aβ from the brain to be associated with damaged BBB integrity and cerebrovascular dysfunction [172]. The BBB protective activity of RES by changing Aβ homeostasis has been observed in several animal models [173].
In rat models of AD, wherein ageing was introduced using a+ D-galactose (D-gal); RES showed improvement by lowering the levels of insoluble Aβ42 thereby maintaining BBB integrity by regulating the expression of RAGE gene, Claudin-5, and matrix metalloprotein-9 (MMP-9) [174]. Clinical trials conducted on AD patients have also confirmed the beneficial effects of RES which significantly restores BBB integrity attributed to MMP-9 reduction. Besides this, it also induces adaptive immune response by promoting brain’s resilience to amyloid deposition in AD patients [175]. These effects lead to decrease in levels of harmful immune molecules consequently reducing BBB infiltration [176] were caused due to reduction in MMP-9. Through genomic studies, it has been identified that LRP1 expression is regulated in AD patients and therefore can be considered as a therapeutic target in AD [177]. In transgenic female rats, RES administration showed increase in LRP1 levels besides unaltered mRNA levels. It is also seen to stabilize and regulate transthyretin by binding with Aβ thereby stopping its aggregation and toxicity resulting in elevation of LRP1 levels [178]. Cytotoxicity of Aβ fibrillar oligomer is also mediated by LRP1 through binding with prion proteins (PrPC). Nonetheless, in SH-SY5Y cells, reduction of PrPC and LRP1 is witnessed soon after RES binding due to change in its conformation [179]. In AD patients, increased expression of RAGE is witnessed. RES through its anti-RAGE activity in the vascular cells counteracts AD pathology [180]. Some reports propose AD as type 3 diabetes, explaining it as a clinical state showing primary effects involving insulin resistance in brain region. Mouse based models confirm the above statement where subjects suffering from type 2 diabetes exhibited decreased expression of RAGE in liver and kidney upon treatment with RES [181]. This is indeed an important finding highlighting the role of RAGE in AD pathology and may be useful in the development of drug targets for Aβ mediated neurological disorders [181].
Resveratrol-mediated Amyloid Plaque Disruption
AD pathology necessarily consists of cholinergic losses in the brain as its characteristic feature besides activation of microglial cells as a result of Aβ aggregation [182]. RES not only affects homeostasis of Aβ but also at times causes aggregation of low molecular weight oligomers into high molecular weight oligomers disturbing the existing Aβ aggregation [178,183]. In a recently concluded study, RES exhibited direct binding to conformers of Aβ, which includes both monomeric and fibrillar forms [184]. Indirect inhibitory effects of RES includes binding between Aβ and Transthyretin which helps in stabilizing Aβ oligomers structure which in turn prevents amyloid plaque aggregation and there by providing therapeutic means for AD [178,185].
Protective role of Resveratrol in Parkinsonian Pathology
AD and PD are among the most common age related neurodegenerative disorders, where oxidative stress and inflammation play determining role [186]. In population aged sixty five and above, rate of occurrence of AD is close to 25% in developed countries [187]. Despite the fact that origin of PD is yet to be accessed; however, several studies declare a number of genetic and environmental factors which significantly influences PD development [188]. Genomic studies have helped gained insights into the cause for PD development; for instance it is now known that mutations/over-expression of α synaptic gene results in the autosomal dominant Parkinsonian syndrome [189].
As per clinical findings, PD can be explained as a progressive neurodegenerative disorder, causing neuronal death in brain and spinal cord regions along with behavioural dysfunctions like tremor, muscle rigidity, and slower movement [190]. In extreme cases, PD may lead to complete loss of movement and muscle activity which is primarily caused due to degeneration of dopaminergic neurons in substantia nigra. In all PD incident accumulation of intra-cytoplasmic Lewy body (aggregates of α-synuclein and ubiquitin) is observed [191]. At this juncture, monoamine oxidase (MAO) has reported to inactivate dopamine synthesis. Moreover, excessive hydrogen peroxide is released in this process [192]. To cope up with this situation, uninterrupted detoxification is required by intracellular antioxidant system. Even though apoptosis is believed to be the primary reason behind decreased dopamine levels nevertheless, the idea remains controversial till date [193]. Oxidative and nitration stress are the major markers of AD and PD [194]. Even though, iNOS activation by nitric oxide and peroxynitrite is well-known; however, origin of harmful oxygen radicals are believed to be produced out of indirect biochemical changes caused due to high iron concentration besides altered mitochondrial function and antioxidant pathways. The above stated mechanism of action is believed to be the reason behind PD development [193]. With more enhanced capabilities along with a coherent source of free radical generation as compared to ATP reduction; mitochondrial complex I inhibition contributes extensively towards oxidative stress in the brain ultimately resulting in the occurrence of neurological diseases [194]. Administration of RES up-regulates PGC-1 and SIRT1-MAPK pathway, which considerably improves mitochondrial respiratory function and could be useful in determining future therapeutic applications [195]. In subsequent steps, the process of mitochondrial biosynthesis is observed to increase mitochondrial function (Figure 2).
PD is always correlated with ATP attenuation and internal flow of calcium ions. Calcium ions generation is mediated through mediators like MAO, produced as a result of dopamine metabolism through ROS in astrocytes [174]. Later, elevation in metabolic stress is observed due to movement of calcium ions into the cell, which is adjusted by ATP pump action that restores calcium homeostasis [150]. RES has shown significant activity in the regulation of calcium levels consequently, retarding PD progression [151].
As per experimental evidences, PD induced animals when administered with RES showed upregulation of antioxidant status and reduced loss of dopamine. Moreover, RES showed 60% hydrogen peroxide (H2O2) clearance activity when administered at 100 μg/mL dosage. Besides, RES is also known to increase SIRT1 levels which prevent accumulation of large amounts of α-synuclein [196]. SIRT1 is found to be useful in generation of heat shock factor 1 (HSF1); which soon after activation and deacetylation affects several molecules and chaperone proteins including transcription of heat shock protein 70 (HSP70) [197]. HSP70 is not only beneficial in maintaining cellular protein balance but also prevents aggregation of malfunctioning proteins. Contemporary research on dopaminergic neuronal cells reveals that administration of GSK-3β inhibitor prevents injury related emergencies by averting phosphorylation of α-synuclein [198]. GSK-3β being a potent mediator of PD pathogenesis; explains how RES administration could prove useful in regulating PD pathogenesis, as is evident from in vitro studies [199].
Protective role of Resveratrol in Huntington disease pathology
Huntington disease (HD) is another disorder of the nervous system caused due to an inherited autosomal dominant mutation in the genome. Disease markers of HD are observed as decrease of striatal neurons progressively causing cognitive impairment along with abnormal body movement [200]. HD is believed to be caused due to repeated duplication of HTT at the N-terminal end in an unstable tri-nucleotide of CAG gene [201]. Functional defects in neurons are pathological manifestation of HD which results in accumulation of toxic aggregates in neurons [202]. Related studies revealed that potent electron transport chain inhibition activity by 3-nitropropionic acid (3-NPA) induces the phenotypic symptoms of HD. RES is known to effectively inhibit activity of 3-NPA and related toxic properties by minimising the production of neurotoxins derivatives consequently [203]. Motor and cognitive functional improvements are seen as a result of inhibition of cyclooxygenase I (COX I) induced by 3-NPA averting neuronal injury [140].
In HD, elevated oxidative stress results in mitochondrial dysfunction due to nerve degeneration. M-HTT besides initiating nerve degeneration leads to programmed cell death. Nonetheless, m-HTT activity could be prevented through activation of P53 in nerve cells. Study on mouse models with HD indicated that cell dysfunction was seen with the expression of p53 gene [204]. In spite of being unaffected phenotypically, it may cause serious impairment of cell division. Removal of acetyl group from the p53 gene has been reported to reduce its toxic functionality at the end phase of apoptosis. RES is found to be effective in the deacetylation of p53 through activation of p53 gene subsequently reducing M-HTT induced cellular toxicity [205]. The protective effects of RES are controlled depending upon the extent of SIRT1 expression, indirectly regulating anti-toxic effects [206-208]. Mitochondrial oxidation has been seen to involve majorly in p53 mediated HD regulation; SIRT1 activation by RES allows cellular apoptosis to the cells suffering from abnormal energy metabolism. Being resistant to oxidation, RES enters into the mitochondria and restores its activity by assisting the expression of SIRT1-PGC1α passage, which in turn controls ROS mediated changes, modification of antioxidant enzyme activity [209].
Antimicrobial effects of resveratrol
RES being a naturally obtained compound, produced by some plants attributed to several varied stimuli. In the past few years, in-depth research has been carried out on the structure, properties and functions of RES. Findings suggest that RES has tremendous capacity in the prevention and treatment of a wide variety of conditions, including cardiovascular diseases, cancer, and in regulation of fungal, bacterial and viral infections [41,210]. RES inhibits replication of both type 1 and type 2 herpes simplex virus (HSV) through inhibition of an early step in the virus replication cycle, thereby controlling the activity of RES against viral infections [211]. Experimental studies by Murakami & coworkers, conducted on animal’s skin treated with RES showed no prominent dermal toxicity, such as erythema, scaling, crusting, lichenification, or excoriation confirming its antimicrobial activity [212].
Resveratrol and estrogens
Hormones responsible for sexual and reproductive development comprising the estrogen response system are immensely modulated and attributed to RES action [213]. This has been experimentally validated through mechanism based studies on different animal model [214,215]. In the doctoral thesis by Savchuk I., the subjects were classified into subgroups according to dosages of trans-RES in 10 g/L of carboxymethylcellulose for 90 days. Simultaneously, control group received only carboxymethylcellulose during this period. Findings include, unchanged testis weight, reduced seminiferous tubules diameter, and prominent increase in tubular density [216]. Apart from these, serum concentration of testosterone, gonadotropins, etc. were found to be significantly increased upon RES treatment [216]. These evidences indicate that RES might serve useful in enhancing reproductive hormone production through stimulation of hypothalamic-pituitary-gonadal axis, without any adverse effects.
Efficacy of resveratrol as a therapeutic drug: Contemporary and future prospects
Over the years, clinical research has helped us in understanding the association between accumulation of different intercellular proteins (like Aβ, α-synuclein etc.) having crucial role in neurological diseases [146]. In this regard, it becomes exceedingly necessary to understand the properties of RES as a therapeutic drug for AD and other similar protein aggregation mediated disease treatment. It is now impeccable for RES to be considered as the primary choice for herbal treatment of neurological diseases like AD [131]. Major therapeutic effects of RES include its role in Aβ production, Aβ clearance, BBB dysfunction, and amyloid plaque disruption (Figure 2) [163]. Additionally, apart from regulation of Aβ homeostasis in brain, RES is also useful in reducing oxidative stress [70]. RES indirectly shows strong Aβ-induced anti-oxidative and anti-inflammatory effects [43]. These benefits strongly advocate the possible therapeutic effects of RES in the treatment of many neurological disorders. Clinical trials with RES reported that it is having strong tolerance towards toxicity besides easy penetration of BBB [176]. However, it exhibits low oral bioavailability and has less clinical efficacy due to extensive metabolism and rapid excretion by our body [27]. In spite of these hindrances, RES attracts researchers as an alternative medicine to cope up with several neurological complications. Still, research and studies are the urgent need of time to explore robust use of RES in clinical perspective.
Resveratrol analogs in AD treatment
Through extensive research, it is known that analogues of RES show better efficacy, stability, and bioavailability than RES and are presently being tested on a number of neurodegenerative disorders [13,72,143]. Therapeutic effects of RES analogues like compounds 5d, a3, 5-dimethoxyl derivatives were seen both in vivo as well as in vitro [217,218]. These compounds can easily cross the BBB and were potent inhibitors of Aβ42 aggregation, structural disintegration, which renders low neurotoxicity [173,174]. Piceatannol, an analogue of RES found in red wine, gives protection against neuronal cell death by interacting with the accumulation of ROS in PC12 cell lines. Pterostilbene, a stilbenoid chemically related to RES is effective in controlling cell stress besides showing cognitive benefits which are associated with peroxisome proliferator-activated receptor alpha (PPAR) protein expression [219]. Through these findings, it is believed that RES analogues could be useful in the treatment of AD and similar neurodegenerative disorders.
Interaction of resveratrol with drugs
When RES administered in combination with anticoagulant agents such as warfarin (Coumadin) [220], antiplatelet drugs, and non-steroidal anti-inflammatory drugs viz. aspirin, ibuprofen [221,222], etc, it showed strong anti-inflammatory and anti-oxidative properties. Reports regarding cytochrome P450 3A4 (CYP3A4) inhibiting activity of RES has been confirmed through in vitro studies [223]. However, the same has not yet been confirmed in humans. Nonetheless, administration of high levels of RES increases the toxicity of drugs (including drugs which are Inhibitors of enzyme HMG-CoA reductase, calcium channel antagonists, anti-arrhythmic agents, HIV proteases, certain immunosuppressant and erectile dysfunction treating drugs) which undergo first-pass metabolism by CYP3A4 [224,225].
Possible adverse effects
Long term effects of RES treatment are unclear however, existing literature talks about regulation of growth potential of human breast cancer cells on account of its chemical structure which is similar to a phytoestrogen. It is also known to have a strong topoisomerase inhibition property as seen in similar polyphenolic compounds exhibiting similarity to drugs like doxorubicin and etoposide which are used in cancer treatment [226-228].
Possible mechanism of therapeutic action of RES
Activity of RES is observed to have several steps involved in its mechanism of action; the initial step being bioactivity involving transfer RNA (tRNA) [229,230]. The tRNAs are amino acid transporters that facilitate protein synthesis. Tyrosyl-tRNA synthetase (TyrRS) is specifically targeted by RES, out of all available tRNA synthetases owing to the structural similarities with tyrosine [229]. While under stress, RES is released in large amount which binds with the available TyrRS replacing tyrosine. Resultant RES-TyrRS complex is thereafter transported to the cell nucleus by activating Poly (ADP-ribose) polymerase 1 (PARP-1) expression [231,232]. Being an important stress response protein, it activates a network of genes useful in protection against stress induced damages to the cell simultaneously assisting in DNA repair [233]. Examples of effectors genes activated by PARP-1 includes; SIRT-6, p53 tumour suppressor gene, and FOXO3A [234].
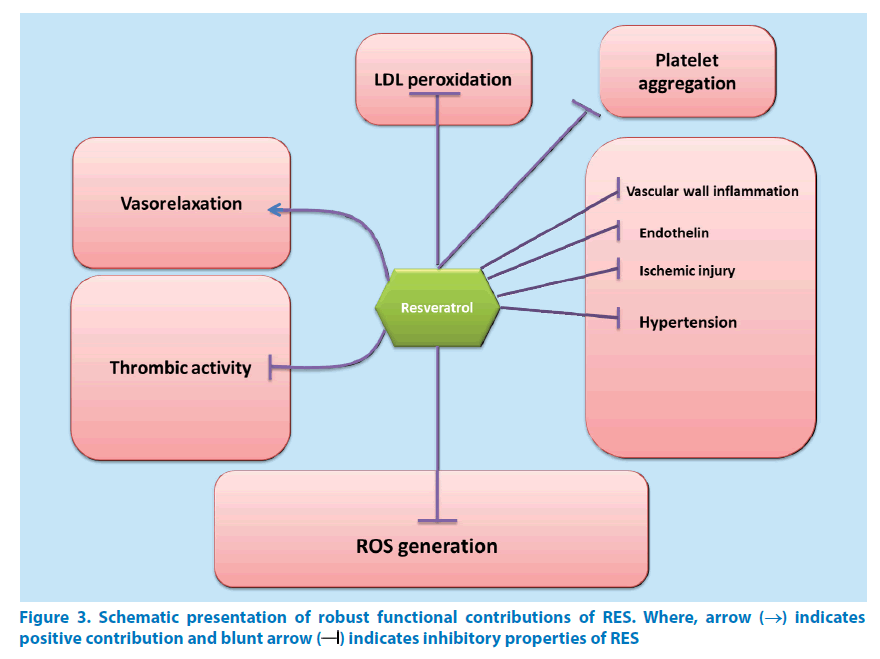
RES is found to be effective against enzymes of sirtuin family especially, SIRT1 enzyme [112,206,208]. For some proteins, it is necessary to have certain site specific amino acids for activation to take place. Activation events like this demonstrate participation of sirtuin-activating compounds (STACs) which make the activation event highly specific and precise [235]. Replacement of amino acid from any site renders the STACs unresponsive and unfit for activation. Likewise, point mutations or lack of point specific amino acid in the enzyme substrate blocks STAC action as well. Clinical experiments on species below humans as per taxonomy have confirmed the role of SIRT-1 in longevity promotion [30,236]. Effects shown by SIRT-1 are observed as improvements in oxidative dephosphorylation as well as mitochondrial aerobic capacity. It is also indispensable for energy regulation and homeostasis. At doses greater than dietary intake, RES activates SIRT-1 in animals. Low bioavailability of RES explains its limitations towards effective activity [27]. Nevertheless, effectiveness of TyRS-RES complex is observed even at much lower concentrations [237] which are useful in treating many disorders viz. stress, inflammation, ageing, cancer, cardiovascular and other similar diseases (Figure 3).
Conclusion and future direction
RES is having high potency to be a drug or candidate drug for the therapy of various anomalies owing to its exceptional antioxidant and anti-inflammatory properties. Several in vivo and in vitro studies have indicated that, RES is capable of regulating several cytotoxic pathways and thereby drags the cellular fate towards cure and/or improvement. RES activities in major neurological disease pathologies are notable in this regard. Especially in AD pathology, RES has shown better therapeutic efficacy. RES also have been reported to be effective in PD and HD pathologies. Direct or indirect pathways of inhibition of Aβ-mediated toxicity have been experimentally validated. RES has also been reported with its anti-aging effect. It is notable that, therapeutic treatment of aging includes mainly, antioxidant and anti-inflammatory treatment, which also serves as the first line of defense. Moreover, research of last few decades have shown efficacious use of RES in the therapeutic means of depression, cognition, stress, major injuries, skin care, aging and related neurological disorders in different animal model systems. Such experimental finding gives us hope that, in the coming days, research work being carried with RES would find novel therapeutic application in the treatment and benefit of elderly patients. However, clinical success of RES administration is equivocal. Frequent failure trials and adverse side effects from RES administration have restricted its use as an effective drug. Such inappropriate druggability of RES is due to drug stability, bioavailability, and non-target side-effects. Therefore, future therapeutics with RES must consider all the assertive research aspects which could improve the drug stability, increase the bioavailability minimize side-effects, and novel drug delivery systems. Together, such research direction could overcome the hindrance of RES use and could provide greater success in clinical use.
Conflict of interest
None
References
- Choudhury A, Chakraborty I, Banerjee TS et al. Efficacy of Morin as a Potential Therapeutic Phytocomponent: Insights into the Mechanism of Action. Int. J. Med. Res. Heal. Sci. 6(11), 175-194 (2017).
- Hui ZG, Zhou XW, Li RJ et al. Studies on the extraction process of total flavonoids in Radix puerariae and their hypoglycemic effect in mice. Biomed. Res. 26(1), 51-54 (2015).
- De Smet PAGM. Drug therapy: Herbal remedies. N. Engl. J. Med. 347, 2046-2056 (2002).
- Urquiaga I, Leighton F. Plant Polyphenol Antioxidants and Oxidative Stress. Biol. Res. 33(2), 55-64 (2000).
- Hassanalilou T, Payahoo L, Shahabi P et al. The protective effects of Morus nigra L. leaves on the kidney function tests and kidney and liver histological structures in streptozotocin-induced diabetic rats. Biomed. Res. 28(14), 6113-6118 (2017).
- Lee CH, Huang GC, Chen CY. Bioactive compounds from natural product extracts in Taiwan cosmeceuticals-mini review. Biomed. Res. 28(15), 6561-6566 (2017).
- Ma SH, Zhang LL JQ. Protective effect of bioflavonoid morin on Cadmium induced oxidative neuropathy. Biomed. Res. 28(3), 1148-54 (2017).
- Lee CH, Huang GC. Bioactive compounds from natural product extracts in Taiwan cosmeceuticals-Mini review. Biomed. Res. 28(1), 6561-6566 (2017).
- Keylor MH, Matsuura BS, Stephenson CRJ. Chemistry and biology of resveratrol-derived natural products. Chem. Rev. 115(17), 8976-9027 (2015).
- Cichewicz RH, Kouzi SA, Atta U. Resveratrol oligomers: Structure, chemistry, and biological activity. Stud. Nat. Prod. Chem. 26(1), 507-579 (2002).
- Rimando AM, Kalt W, Magee JB et al. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 52(15), 4713-4719 (2004).
- Lyons MM, Yu C, Toma RB et al. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 51(20), 5867-5870 (2003).
- Rimando AM, Barney DL. Resveratrol and naturally occurring analogues in vaccinium species. In: Acta Horticulturae. 137-143 (2005).
- Frémont L. Biological effects of resveratrol. Antioxid. Redox Signal. 3(6), 1041-1064 (2001).
- Oomen CA, Farkas E, Roman V et al. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front. Aging Neurosci. (2009).
- de Ligt M, Timmers S, Schrauwen P. Resveratrol and obesity: Can resveratrol relieve metabolic disturbances? Biochim. Biophys. Acta. 1852(6), 1137-1144 (2015).
- Wight RD, Tull CA, Deel MW et al. Resveratrol effects on astrocyte function: Relevance to neurodegenerative diseases. Biochem. Biophys. Res. Commun. 426(1), 112-115 (2012).
- Gresele P, Cerletti C, Guglielmini G et al. Effects of resveratrol and other wine polyphenols on vascular function: An update. J. Nutr. Biochem. 22(3), 201-211 (2011).
- Baile CA, Yang JY, Rayalam S et al. Effect of resveratrol on fat mobilization. Ann. N. Y. Acad. Sci. 1215(1), 40-47 (2011).
- Nemoto H, Katagiri A, Kamiya M et al. Synthesis and evaluation of water-soluble resveratrol and piceatannol via BGLation. Bioorganic Med. Chem. Lett. 22(15), 5051-5054 (2012).
- Chung IM, Park MR, Chun JC et al. Resveratrol accumulation and resveratrol synthase gene expression in response to abiotic stresses and hormones in peanut plants. Plant. Sci. 164(1), 103-109 (2003).
- Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am. J. Enol. Vitic. 43(1), 49-52 (1992).
- Holthoff JH, Woodling KA, Doerge DR et al. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem. Pharmacol. 80(8), 1260-1265 (2010).
- Takaoka M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. J. Chem. Soc. Japan. 60, 1090-1100 (1939).
- Xu B, Chen S, Luo Y et al. Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS. One. 6(4) (2011).
- Hartman J, Frishman WH. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 22(3), 147-151 (2014).
- Walle T, Hsieh F, DeLegge MH et al. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 32(12), 1377-1382 (2004).
- Janle EM, Lila MA, Grannan M et al. Pharmacokinetics and tissue distribution of 14C-labeled grape polyphenols in the periphery and the central nervous system following oral administration. J. Med. Food. 13(4), 926-33 (2010).
- Mullin GE. Red wine, grapes, and better health-resveratrol. Nutr. Clin. Pract. 26(6), 722-723 (2011).
- Das DK, Mukherjee S, Ray D. Resveratrol and red wine, healthy heart and longevity. Heart. Fail. Rev. 15(5), 467-477 (2010).
- Burns J, Yokota T, Ashihara H et al. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 50(11), 3337-3340 (2002).
- Sanders TH, McMichael RW, Hendrix KW. Occurrence of resveratrol in edible peanuts. J. Agric. Food Chem. 48(4), 1243-1246 (2000).
- Hurst WJ, Glinski JA, Miller KB et al. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J. Agric. Food Chem. 56(18), 8374-8378 (2008).
- Jeandet P, Sbaghi M, Bessis R et al. The potential relationship of stilbene (resveratrol) synthesis to anthocyanin content in grape berry skins. Vitis. 34(2), 91-94 (1995).
- Pereira GE, Gaudillere JP, Pieri P et al. Microclimate influence on mineral and metabolic profiles of grape berries. J. Agric. Food Chem. 54(18), 6765-6775 (2006).
- Goldberg DM, Karumanchiri A, Ng E et al. Direct Gas Chromatographic-Mass Spectrometric Method To Assay cis-Resveratrol in Wines: Preliminary Survey of Its Concentration in Commercial Wines. J. Agric. Food Chem. 43(5), 1245-1250 (1995).
- Sobolev VS, Cole RJ. Trans-resveratrol content in commercial peanuts and peanut products. J. Agric. Food Chem. 47(4), 1435-1439 (1999).
- Stervbo U, Vang O, Bonnesen C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food. Chem. 101(2), 449-457 (2007).
- Amri A, Chaumeil JC, Sfar S et al. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release. 158(2), 182-193 (2012).
- Summerlin N, Soo E, Thakur S et al. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 479(2), 282-290 (2015).
- Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer. 21(3) (2014).
- Szkudelski T, Szkudelska K. Anti-diabetic effects of resveratrol. Ann. N. Y. Acad. Sci. 1215(1), 34-39 (2011).
- Aldawsari FS, Aguiar RP, Wiirzler LAM et al. Anti-inflammatory and antioxidant properties of a novel resveratrol-salicylate hybrid analog. Bioorganic. Med. Chem. Lett. 26(5), 1411-1415 (2016).
- Tomé-Carneiro J, Larrosa M, González-Sarrías A et al. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 19(34), 6064-6093 (2013).
- Ronksley PE, Brien SE, Turner BJ et al. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 342, d671 (2011).
- Catalgol B, Batirel S, Taga Y et al. Resveratrol: French paradox revisited. Front. Pharmacol. (2012).
- Lippi G, Franchini M, Favaloro EJ et al. Moderate red wine consumption and cardiovascular disease risk: Beyond the French Paradox. Semin. Thromb. Hemost. 36(1), 59-70 (2010).
- Salvamani S, Gunasekaran B, Shaharuddin NA et al. Antiartherosclerotic effects of plant flavonoids. Biomed. Res. Int. 2014 (2014).
- Gronbaek M, Becker U, Johansen D et al. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann. Intern. Med. 133(6), 411-419 (2000).
- Klatsky AL, Friedman GD, Armstrong MA et al. Wine, liquor, beer, and mortality. Am. J. Epidemiol. 158(6), 585-595 (2003).
- Renaud SC, Guéguen R, Siest G et al. Wine, beer, and mortality in middle-aged men from Eastern France. Arch. Intern. Med. 159(16), 1865-1870 (1999).
- Mukamal KJ, Conigrave KM, Mittleman M et al. Roles of drinking pattern and type of alchohol consumed in coronary heart disease in men. N. Engl. J. Med. 348, 109-118 (2003).
- Rimm EB, Klatsky A, Grobbee D et al. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits. BMJ. 312(7033), 731-736 (1996).
- Wannamethee SG, Shaper AG. Type of alcoholic drink and risk of major coronary heart disease events and all-cause mortality. Am. J. Public Health. 89(5), 685-690 (1999).
- Johansen D, Friis K, Skovenborg E et al. Food buying habits of people who buy wine or beer: Cross sectional study. Br. Med. J. 332(7540), 519-521 (2006).
- Ekshyyan VP, Hebert VY, Khandelwal A et al. Resveratrol inhibits rat aortic vascular smooth muscle cell proliferation via estrogen receptor dependent nitric oxide production. J. Cardiovasc. Pharmacol. 50(1), 83-93 (2007).
- Khandelwal AR, Hebert VY, Dugas TR. Essential role of ER-alpha-dependent NO production in resveratrol-mediated inhibition of restenosis. Am. J. Physiol. Heart Circ. Physiol. 299(5), H1451-H1458 (2010).
- Yang YM, Chen JZ, Wang XX et al. Resveratrol attenuates thromboxane A2receptor agonist-induced platelet activation by reducing phospholipase C activity. Eur. J. Pharmacol. 583(1), 148-155 (2008).
- Shen MY, Hsiao G, Liu CL et al. Inhibitory mechanisms of resveratrol in platelet activation: Pivotal roles of p38 MAPK and NO/cyclic GMP. Br. J. Haematol. 139(3), 475-485 (2007).
- Lerman A, Zeiher AM. Endothelial function: Cardiac events. Circulation. 111(3), 363-368 (2005).
- Wang Z, Zou J, Cao K et al. Dealcoholized red wine containing known amounts of resveratrol suppresses atherosclerosis in hypercholesterolemic rabbits without affecting plasma lipid levels. Int. J. Mol. Med. 16(4), 533-540 (2005).
- Maree AO, Fitzgerald DJ. Variable platelet response to aspirin and clopidogrel in atherothrombotic disease. Circulation. 115(16), 2196-2207 (2007).
- Lance EI, Sreenivasan AK, Zabel TA et al. Aspirin use in sturge-weber syndrome: Side effects and clinical outcomes. J. Child Neurol. 28(2), 213-218 (2013).
- Klinge CM, Wickramasinghe NS, Ivanova MM et al. Resveratrol stimulates nitric oxide production by increasing estrogen receptor -Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 22(7), 2185-2197 (2008).
- Stef G, Csiszar A, Lerea K et al. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J. Cardiovasc. Pharmacol. 48(2), 1-5 (2006).
- Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 341(8852), 1103-1104 (1993).
- Frémont L, Belguendouz L, Delpal S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life. Sci. 64(26), 2511-2521 (1999).
- Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 72(11), 1439-1452 (2006).
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 5(6), 493-506 (2006).
- Leonard SS, Xia C, Jiang BH et al. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 309(4), 1017-1026 (2003).
- Patel KR, Scott E, Brown VA et al. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 1215(1), 161-169 (2011).
- Aggarwal BB, Bhardwaj A, Aggarwal RS et al. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer. Res. 24(5A), 2783-2840 (2004).
- Spanier G, Xu H, Xia N et al. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 60 Suppl 4, 111-116 (2009).
- Kode A, Rajendrasozhan S, Caito S et al. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 294(3), L478-88 (2008).
- Wang H, Yang YJ, Qian HY et al. Resveratrol in cardiovascular disease: What is known from current research? Heart. Fail. Rev. 17(3), 437-448 (2012).
- Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug. Rev. 22(3), 169-188 (2004).
- Filip V, Plocková M, Šmidrkal J et al. Resveratrol and its antioxidant and antimicrobial effectiveness. Food. Chem. 83(4), 585-593 (2003).
- Subbaramaiah K, Chung WJ, Michaluart P et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J. Biol. Chem. 273(34), 21875-21882 (1998).
- Hao HD, He LR. Mechanisms of cardiovascular protection by resveratrol. J. Med. Food. 7(3), 290-298 (2004).
- Buluc M, Demirel-Yilmaz E. Resveratrol decreases calcium sensitivity of vascular smooth muscle and enhances cytosolic calcium increase in endothelium. Vascul. Pharmacol. 44(4), 231-237 (2006).
- Park JS, Kim KM, Kim MH et al. Resveratrol inhibits tumor cell adhesion to endothelial cells by blocking ICAM-1 expression. Anticancer. Res. 29(1), 355-362 (2009).
- Petrat F, De Groot H. Protection against severe intestinal ischemia/reperfusion injury in rats by intravenous resveratrol. J. Surg. Res. 167(2), (2011).
- Kundu JK, Surh YJ. Molecular basis of chemoprevention by resveratrol: NF-kB and AP-1 as potential targets. Mutat. Res. Fundam. Mol. Mech. Mutagen. 555(1-2), 65-80 (2004).
- Tung BT, Rodríguez-Bies E, Talero E et al. Anti-inflammatory effect of resveratrol in old mice liver. Exp. Gerontol. 64, 1-7 (2015).
- Turner MD, Nedjai B, Hurst T et al. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. Mol. Cell. Res. 1843(11), 2563-2582 (2014).
- Xia Feng, Ji Hong Xue, Kun Xia Xie et al. Beneficial effect of Mangiferin against sleep deprivation-induced neurodegeneration and memory impairment in mice. Biomed. Res. 28(2), 769-777 (2017).
- Sasikumar K, Adalarasu K. Analysis of physiological signal variation between autism and control group in south indian population. Biomed. Res. 26(3), 525-529 (2015).
- Chang MY, Liu CM, Shieh DE, Chen C. Evaluation and analysis of phytochemical antioxidant capacity. Biomed. Res. 28(14), 6431-6434 (2017).
- Han CL, Fu R LW. Synthesis and antidementia effects of a new Zn (II) coordination polymer. Biomed. Res. 27(4), 1237-1239 (2016).
- Lee CH, Huang GC CC. Bioactive compounds from natural product extracts in Taiwan cosmeceuticals-Mini review. Biomed. Res. 28(15), 6561-6566 (2017).
- Priya MS KNG. Modified emotion recognition system to study the emotion cues through thermal facial analysis. Biomed. Res. 28(19), 1-6 (2017).
- Menzies KJ, Singh K, Saleem A et al. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J. Biol. Chem. 288(10), 6968-6979 (2013).
- Camins A, Vilaplana J. Sirtuin and resveratrol. In Vivo (Brooklyn). 329-340 (2009).
- Davinelli S, Sapere N, Visentin M et al. Enhancement of mitochondrial biogenesis with polyphenols: Combined effects of resveratrol and equol in human endothelial cells. Immun. Ageing. 10(1), (2013).
- Sadowska-Bartosz I, Bartosz G. Effect of antioxidants supplementation on aging and longevity. Biomed. Res. Int. 2014, (2014).
- Barger JL, Kayo T, Vann JM et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS. One. 3(6), (2008).
- Imai S ichiro, Guarente L. NAD+ and sirtuins in aging and disease. Trends. Cell. Biol. 24(8), 464-471 (2014).
- Kimura Y, Okuda H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. J. Nutr. 131(6), 1844-9 (2001).
- Tang Q, Li G, Wei X et al. Resveratrol-induced apoptosis is enhanced by inhibition of autophagy in esophageal squamous cell carcinoma. Cancer. Lett. 336(2), 325-337 (2013).
- Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. 2, 409-419 (2009).
- Wu Y, Liu F. Targeting mTOR: Evaluating the therapeutic potential of resveratrol for cancer treatment. 1, 1032-1038 (2013).
- Jang M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Sci. (80). 275(5297), 218-220 (1997).
- Jang M, Cai L, Udeani GO et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Sci. 275(5297), 218-220 (1997).
- Lu R, Serrero G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human. 304, 297-304 (1999).
- Park D, Jeong H, Lee MN et al. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 6 (2016).
- Liu M, Liu F. Resveratrol inhibits mTOR signaling by targeting DEPTOR. Commun. Integr. Biol. 4(4), 382-384 (2011).
- Peterson TR, Laplante M, Thoreen CC et al. DEPTOR Is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 137(5), 873-886 (2009).
- Li Z, Shimada Y, Kawabe A et al. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by JTE-522, a selective COX-2 inhibitor. Carcinogenesis. 22(4), 547-551 (2001).
- Schneider Y, Duranton B, Gossé F et al. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr. Cancer. 39(1), 102-107 (2001).
- Opipari AW, Tan L, Boitano AE et al. Resveratrol-induced autophagocytosis in ovarian cancer cells. (20), 696-703 (2004).
- Dabrosin C, Garvin S, Karin O. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. 231, 113-122 (2006).
- Howitz KT, Bitterman KJ, Cohen HY et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 425(6954), 191-196 (2003).
- Baur JA, Pearson KJ, Price NL et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 444(7117), 337-342 (2006).
- Borriello A, Bencivenga D, Caldarelli I et al. Resveratrol and cancer treatment: is hormesis a yet unsolved matter? 5384-5393 (2013).
- Scarlatti F, Sala G, Somenzi G et al. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. FASEB J. 17(15), 2339-41 (2003).
- Signorelli P, Munoz-olaya JM, Gagliostro V et al. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer. Lett. 282(2), 238-243 (2009).
- Bishayee A, Politis T, Darvesh AS. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer. Treat. Rev. 36(1), 43-53 (2010).
- Huang C, Ma WY, Goranson A et al. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis. 20(2), 237-242 (1999).
- Niles RM, McFarland M, Weimer MB et al. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer. Lett. 190(2), 157-163 (2003).
- Jha RK, Ma Q, Sha H et al. Emerging role of resveratrol in the treatment of severe acute pancreatitis. Front. Biosci. (Schol. Ed). 2, 168-175 (2010).
- Shih A, Davis FB, Lin HY et al. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J. Clin. Endocrinol. Metab. 87(3), 1223-1232 (2002).
- Tili E, Michaille JJ, Alder H et al. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem. Pharmacol. 80(12), 2057-2065 (2010).
- Panaro MA, Carofiglio V, Acquafredda A et al. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br. J. Nutr. 108(9), 1623-1632 (2012).
- Delmas D, Rebe C, Lacour S et al. Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J. Biol. Chem. 278(42), 41482-41490 (2003).
- Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 16(8), 449-466 (2005).
- Varoni EM, Lo Faro AF, Sharifi-Rad J et al. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 3 (2016).
- Qin W, Zhang K, Clarke K et al. Methylation and miRNA effects of resveratrol on mammary tumors vs. normal tissue. Nutr. Cancer. 66(2), 270-277 (2014).
- Venkatadri R, Muni T, Iyer AKV et al. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell. Death. Dis. 7, e2104 (2016).
- Albani D, Polito L, Signorini A et al. Neuroprotective properties of resveratrol in different neurodegenerative disorders. BioFactors. 36(5), 370-376 (2010).
- Richard T, Pawlus AD, Iglésias ML et al. Neuroprotective properties of resveratrol and derivatives. Ann. N. Y. Acad. Sci. 1215(1), 103-108 (2011).
- Vingtdeux V, Dreses-Werringloer U, Zhao H et al. Therapeutic potential of resveratrol in Alzheimer’s disease. In: BMC Neuroscience. (2008).
- Hu M, Liu B. Resveratrol via activation of LKB1-AMPK signaling suppresses oxidative stress to prevent endothelial dysfunction in diabetic mice. Clin. Exp. Hypertens. 38(4), 381-387 (2016).
- Van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr. Neurol. Neurosci. Rep. 10(3), 207-214 (2010).
- Deane R, Bell R, Sagare A et al. Clearance of amyloid-β peptide across the blood-brain barrier: implication for therapies in alzheimers disease. CNS. Neurol. Disord. Drug Targets. 8(1), 16-30 (2009).
- O’Brien RJ, Wong PC. Amyloid precursor protein processing and alzheimer’s disease. Annu. Rev. Neurosci. 34(1), 185-204 (2011).
- Tarasoff-Conway JM, Carare RO, Osorio RS et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11(8), 457-470 (2015).
- Nilsson P, Loganathan K, Sekiguchi M et al. Aβ secretion and plaque formation depend on autophagy. Cell Rep. 5(1), 61-69 (2013).
- Gosselet F, Saint-Pol J, Candela P et al. Amyloid-β Peptides, alzheimer’s disease and the blood-brain barrier. Curr. Alzheimer Res. 10(10), 1015-1033 (2013).
- Yoon SS, Jo SA. Mechanisms of amyloid-β peptide clearance: potential therapeutic targets for alzheimer’s disease. Biomol. Ther. 20(3), 245-255 (2012).
- Zhang W, Xiong H, Callaghan D et al. Blood-brain barrier transport of amyloid beta peptides in efflux pump knock-out animals evaluated by in vivo optical imaging. Fluids. Barriers. CNS. 10(1), 13 (2013).
- Ghobeh M, Ahmadian S, Meratan AA et al. Interaction of A(25-35) fibrillation products with mitochondria: Effect of small-molecule natural products. In: Biopolymers. Peptide. Sci. Section. 473-486 (2014).
- Pacheco-Quinto J, Herdt A, Eckman CB et al. Endothelin-converting enzymes and related metalloproteases in Alzheimer’s disease. Adv. Alzheimer’s. Dis. 3, 101-110 (2012).
- Coppa T, Lazzè MC, Cazzalini O et al. Structure-activity relationship of resveratrol and its analogue, 4,4’-dihydroxy-trans-stilbene, toward the endothelin axis in human endothelial cells. J. Med. Food. 14(10), 1173-80 (2011).
- Wyganowska-Świątkowska M, Surdacka A, Skrzypczak-Jankun E et al. The plasminogen activation system in periodontal tissue. Int. J. Mol. Med. 33(4), 763-768 (2014).
- Wu SL, Zhan DM, Xi SH et al. Roles of tissue plasminogen activator and its inhibitor in proliferative diabetic retinopathy. Int. J. Ophthalmol. 7(5), 764-767 (2014).
- Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 11(11), 777-788 (2010).
- Tamagno E, Parola M, Bardini P et al. β-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J. Neurochem. 92(3), 628-636 (2005).
- Yan R, Vassar R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet. Neurol. 13(3), 319-329 (2014).
- Ohno M. Alzheimer’s therapy targeting the β-secretase enzyme BACE1: Benefits and potential limitations from the perspective of animal model studies. Brain. Res. Bull. 126, 183-198 (2016).
- Choi CW, Choi YH, Cha MR et al. In vitro BACE-1 inhibitory activity of resveratrol oligomers from the seed extract of paeonia lactiflora. Planta. Med. 77(4), 374-376 (2011).
- Koukoulitsa C, Villalonga-Barber C, Csonka R et al. Biological and computational evaluation of resveratrol inhibitors against Alzheimers disease. J. Enzyme Inhib. Med. Chem. 31(1), 67-77 (2016).
- Skretas G, Meligova AK, Villalonga-Barber C et al. Engineered chimeric enzymes as tools for drug discovery: Generating reliable bacterial screens for the detection, discovery, and assessment of estrogen receptor modulators. J. Am. Chem. Soc. 129(27), 8443-8457 (2007).
- May PC, Willis BA, Lowe SL et al. The potent BACE1 inhibitor LY2886721 elicits robust central a pharmacodynamic responses in mice, dogs, and humans. J. Neurosci. 35(3), 1199-1210 (2015).
- Vardy ERLC, Hussain I, Hooper NM. Emerging therapeutics for Alzheimer’s disease. Expert. Rev. Neurother. 6(5), 695-704 (2006).
- Zhang WLS. Study on the determination of lactone contents in Ginkgo biloba leaves and their effects in schizophrenia. Biomed. Res. 26(1), 31-36 (2015).
- Abdulazeez SS. Freeze dried strawberry powder ameliorates alloxan induced hyperlipidemia in diabetic rats. Biomed. Res. 26(1), 77-81 (2015).
- Xinxue Gao, Yu Zhang, Xiuzhen Zhang et al. Anti-depressant treatment-related changes in risk factors and their impact on the prognosis of sleep disturbances in patients with depression. Biomed. Res. 28(14), 6435-6440 (2017).
- Lin Wu, Y-ML. Factors associated with Parkinson’s disease patients with hyposmia in Chines e han population: a case-control study. Biomed. Res. 27(2), 513-516 (2016).
- Plaschke K, Kopitz J. In vitro streptozotocin model for modeling Alzheimer-like changes: effect on amyloid precursor protein secretases and glycogen synthase kinase-3. J. Neural Transm. 122(4), 551-557 (2015).
- Acx H, Serneels L, Radaelli E et al. Inactivation of γ‐secretases leads to accumulation of substrates and non‐Alzheimer neurodegeneration. EMBO. Mol. Med. 9(8), 1088-1099 (2017).
- Torres G, Dileo JN, Hallas BH et al. Silent information regulator 1 mediates hippocampal plasticity through presenilin1. Neuroscience. 179, 32-40 (2011).
- He X, Li Z, Rizak JD et al. Resveratrol attenuates formaldehyde induced hyperphosphorylation of tau protein and cytotoxicity in N2a cells. Front. Neurosci. 10, (2017).
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-?? peptides. J. Biol. Chem. 280(45), 37377-37382 (2005).
- Huang YY, Bach ME, Lipp HP et al. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc. Natl. Acad. Sci. 93(16), 8699-8704 (1996).
- Wang W, Liu C, Sun K. Induction of amnion epithelial apoptosis by cortisol via tPA/Plasmin system. Endocrinology. 157(11), 4487-4498 (2016).
- Lijnen HR. Pleiotropic functions of plasminogen activator inhibitor-1. J. Thromb. Haemost. 3(1), 35-45 (2005).
- Konkle B a, Ginsburg D. The addition of endothelial cell growth factor and heparin to human umbilical vein endothelial cell cultures decreases plasminogen activator inhibitor-1 expression. J. Clin. Invest. 82(2), 579-85 (1988).
- Tomé-Carneiro J, Gonzálvez M, Larrosa M et al. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am. J. Cardiol. 110(3), 356-363 (2012).
- Cho KS, Lee EJ, Kwon KJ et al. Resveratrol down-regulates a glutamate-induced tissue plasminogen activator via Erk and {AMPK/mTOR} pathways in rat primary cortical neurons. Food. Funct. 5(5), 951-960 (2014).
- Choudhury A, Rao D, Varma J et al. Cognitive and psychological anomalies in parkinson’s disease: an insight into non-motor characteristic features. Neuropsychiatry. 7(6), 1069-1080 (2017).
- Abbott NJ, Patabendige AAK, Dolman DEM et al. Structure and function of the blood-brain barrier. Neurobiol. Dis. 37(1), 13-25 (2010).
- Jones PB, Adams KW, Rozkalne A et al. Apolipoprotein E: Isoform specific differences in tertiary structure and interaction with amyloid-β in human Alzheimer brain. PLoS. One. 6(1), (2011).
- Saha A, Sarkar C, Singh SP et al. The blood-brain barrier is disrupted in a mouse model of infantile neuronal ceroid lipofuscinosis: Amelioration by resveratrol. Hum. Mol. Genet. 21(10), 2233-2244 (2012).
- Zhao HF, Li N, Wang Q, Cheng XJ et al. Resveratrol decreases the insoluble Aβ1-42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neuroscience. 310, 641-649 (2015).
- Moussa C, Hebron M, Huang X et al. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflammation. 14(1) (2017).
- https://www.sciencedaily.com/releases/2016/07/160727140041.htm
- Abuznait AH, Kaddoumi A. Role of ABC transporters in the pathogenesis of Alzheimers disease. ACS. Chem. Neurosci. 3(11), 820-831 (2012).
- Santos LM, Rodrigues D. Resveratrol administration increases transthyretin protein levels, ameliorating ad features: the importance of transthyretin tetrameric stability. Mol. Med. 22(1), 1 (2016).
- Hu NW, Nicoll AJ, Zhang D et al. MGlu5 receptors and cellular prion protein mediate amyloid-β- facilitated synaptic long-term depression in vivo. Nat. Commun. 5 (2014).
- Ahmed F, Rahman MS. Preliminary assessment of free radical scavenging, thrombolytic and membrane stabilizing capabilities of organic fractions of Callistemon citrinus (Curtis.) skeels leaves. BMC Complement. Altern. Med. 16(1), 1 (2016).
- Khazaei M, Karimi J, Sheikh N et al. Effects of resveratrol on receptor for advanced glycation end products (rage) expression and oxidative stress in the liver of rats with type 2 diabetes. Phyther. Res. 30(1), 66-71 (2016).
- Malm TM, Jay TR, Landreth GE. The evolving biology of microglia in alzheimer’s disease. Neurotherapeutics. 12(1), 81-93 (2015).
- Leclerc S, Garnier M, Hoessel R et al. Indirubins inhibit glycogen synthase kinase-3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 276(1), 251-260 (2001).
- Frozza RL, Bernardi A, Hoppe JB et al. Neuroprotective effects of resveratrol against Aβ administration in rats are improved by lipid-core nanocapsules. Mol. Neurobiol. 47(3), 1066-1080 (2013).
- Ribeiro CA, Oliveira SM, Guido LF et al. Transthyretin stabilization by iododiflunisal promotes amyloid-β peptide clearance, decreases its deposition, and ameliorates cognitive deficits in an Alzheimer’s disease mouse model. J. Alzheimer’s Dis. 39(2), 357-370 (2014).
- Choudhury A, Chakraborty I, Banerjee TS et al. Effect and disease indicative role of inflammation in neurodegenerative pathology: a mechanistic crosstalk of promise and dilemma. Neuropsychiatry. 8(1), 739-749 (2018).
- Goedert M. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Sci. 349(6248) (2015).
- Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson’s disease: Evidence from studies of non-human primates. Nat. Rev. Neurosci. 12(6), 359-366 (2011).
- Schulte C, Gasser T. Genetic basis of Parkinson’s disease: Inheritance, penetrance, and expression. Appl. Clin. Genet. 4, 67-80 (2011).
- Lotharius J, Brundin P. Pathogenesis of parkinson’s disease: Dopamine, vesicles and α-synuclein. Nat. Rev. Neurosci. 3(12), 932-942 (2002).
- Wakabayashi K, Tanji K, Mori F et al. The Lewy body in Parkinson’s disease: Molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology. 494-506 (2007).
- Chaurasiya ND, Ibrahim MA, Muhammad I et al. Monoamine oxidase inhibitory constituents of propolis: Kinetics and mechanism of inhibition of recombinant human MAO-A and MAO-B. Molecules. 19(11), 18936-18952 (2014).
- Naoi M, Riederer P, Maruyama W. Modulation of monoamine oxidase (MAO) expression in neuropsychiatric disorders: genetic and environmental factors involved in type A MAO expression. J. Neural. Transm. 123(2), 91-106 (2016).
- Valko M, Leibfritz D, Moncol J et al. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39(39), 44-84 (2007).
- Lee JH, Moon JH, Kim SW et al. EGCG-mediated autophagy flux has a neuroprotection effect via a class III histone deacetylase in primary neuron cells. Oncotarget. 6(12), 9701-9717 (2015).
- Martin A, Tegla CA, Cudrici CD et al. Role of SIRT1 in autoimmune demyelination and neurodegeneration. Immunol. Res. 61(3), 187-197 (2015).
- Wang N, Zhang F, Yang L et al. Resveratrol protects against L-Arginine-induced acute necrotizingpancreatitis in mice by enhancing SIRT1-mediated deacetylation of p53 and heat shock factor 1. Int. J. Mol. Med. 40(2), 427-437 (2017).
- Ga̧ssowska M, Czapski GA, Pajaķ B et al. Extracellular α-synuclein leads to microtubule destabilization via GSK-3β-dependent tau phosphorylation in PC12 cells. PLoS. One. 9(4) (2014).
- Zeng W, Zhang W, Lu F et al. Resveratrol attenuates MPP+-induced mitochondrial dysfunction and cell apoptosis via AKT/GSK-3β pathway in SN4741 cells. Neurosci. Lett. 637, 50-56 (2017).
- Naia L, Rosenstock TR, Oliveira AM et al. Comparative mitochondrial-based protective effects of resveratrol and nicotinamide in huntington’s disease models. Mol. Neurobiol. 54(7), 5385-5399 (2017).
- Mollersen L, Moldestad O, Rowe AD et al. Effects of anthocyanins on cag repeat instability and behaviour in huntington’s disease r6/1 mice. PLoS. Curr. 8 (2016).
- Leitman J, Ulrich Hartl F, Lederkremer GZ. Soluble forms of polyQ-expanded huntingtin rather than large aggregates cause endoplasmic reticulum stress. Nat. Commun. 4 (2013).
- Kumar P, Padi SSV, Naidu PS et al. Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: Possible neuroprotective mechanisms. Behav. Pharmacol. 17(5-6), 485-492 (2006).
- Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 38(3), 357-366 (1995).
- Zheng S, Koh XY, Goh HC et al. Inhibiting p53 acetylation reduces cancer chemotoxicity. Cancer Res. 77(16), 4342-4354 (2017).
- Beher D, Wu J, Cumine S et al. Resveratrol is not a direct activator of sirt1 enzyme activity. Chem. Biol. Drug Des. 74(6), 619-624 (2009).
- Sin TK, Tam BT, Yung BY et al. Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J. Physiol. 593(8), 1887-1899 (2015).
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 280(17), 17187-17195 (2005).
- Schirmer H, Pereira TCB, Rico EP et al. Modulatory effect of resveratrol on SIRT1, SIRT3, SIRT4, PGC1α and NAMPT gene expression profiles in wild-type adult zebrafish liver. Mol. Biol. Rep. 39(3), 3281-3289 (2012).
- Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann. N. Y. Acad. Sci. 1215(1), 22-33 (2011).
- Campagna M, Rivas C. Antiviral activity of resveratrol. Biochem. Soc. Trans. 38(1), 50-53 (2010).
- Murakami I, Chaleckis R, Pluskal T et al. Metabolism of skin-absorbed resveratrol into its glucuronized form in mouse skin. PLoS One. 9(12), (2014).
- Gehm BD, Mcandrews JM, Chien PY et al. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Physiol. Commun. 94, 14138-14143 (1997).
- Odermatt A, Strajhar P, Engeli RT. Disruption of steroidogenesis: Cell models for mechanistic investigations and as screening tools. J. Steroid Biochem. Mol. Biol. 158, 9-21 (2016).
- Harvey PW, Everett DJ. The adrenal cortex and steroidogenesis as cellular and molecular targets for toxicity: Critical omissions from regulatory endocrine disrupter screening strategies for human health? J. Appl. Toxicol. 23(2), 81-87 (2003).
- Savchuk I. Diverse effects of endocrine-disruptive chemicals on Leydig and adrenocortical cell steroidogenesis in rodents and humans.
- Fulda S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug. Discov. Today. 15(17-18), 757-765 (2010).
- Aldawsari FS, Velázquez-Martínez CA. 3,4′,5-trans-Trimethoxystilbene; A natural analogue of resveratrol with enhanced anticancer potency. Invest. New Drugs. 33(3), 775-786 (2015).
- Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. Rev. Mutat. Res. 750(1), 60-82 (2012).
- Chiba T, Kimura Y, Suzuki S et al. Trans-resveratrol enhances the anticoagulant activity of warfarin in a mouse model. J. Atheroscler. Thromb. 23(9), 1099-1110 (2016).
- Lissa D, Senovilla L, Rello-Varona S et al. Resveratrol and aspirin eliminate tetraploid cells for anticancer chemoprevention. Proc. Natl. Acad. Sci. 111(8), 3020-3025 (2014).
- MacDonald TM, Wei L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet. 361(9357), 573-574 (2003).
- Chan WK, Delucchi AB. Resveratrol, a red wine constituent, is a mechanism-based inactivator of cytochrome P450 3A4. Life. Sci. 67(25), 3103-3112 (2000).
- Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr. Drug Metab. 9(4), 310-322 (2008).
- Chow HHS, Garland LL, Hsu CH et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer. Prev. Res. 3(9), 1168-1175 (2010).
- Pommier Y. DNA topoisomerase I Inhibitors: Chemistry, biology, and interfacial inhibition. Chem. Rev. 109(7), 2894-2902 (2009).
- Demoulin B, Hermant M, Castrogiovanni C et al. Resveratrol induces DNA damage in colon cancer cells by poisoning topoisomerase II and activates the ATM kinase to trigger p53-dependent apoptosis. Toxicol. Vitr. 29(5), 1156-1165 (2015).
- Leone S, Cornetta T, Basso E et al. Resveratrol induces DNA double-strand breaks through human topoisomerase II interaction. Cancer. Lett. 295(2), 167-172 (2010).
- Sajish M, Schimmel P. A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol. Nature. 519(7543), 370-373 (2015).
- Markus MA, Marques FZ, Morris BJ. Resveratrol, by modulating RNA processing factor levels, can influence the alternative splicing of Pre-mRNAs. PLoS. One. 6(12) (2011).
- Deng H, Mi M tian. Resveratrol attenuates Aβ25-35 caused neurotoxicity by inducing autophagy through the TyrRS-PARP1-SIRT1 signaling pathway. Neurochem. Res. 41(9), 2367-2379 (2016).
- Iraha F, Oki K, Kobayashi T et al. Functional replacement of the endogenous tyrosyl-tRNA synthetase-tRNATyrpair by the archaeal tyrosine pair in Escherichia coli for genetic code expansion. Nucleic. Acids. Res. 38(11), 3682-3691 (2010).
- Cao X, Li C, Xiao S et al. Acetylation promotes TyrRS nuclear translocation to prevent oxidative damage. Proc. Natl. Acad. Sci. 114(4), 687-692 (2017).
- Tseng PC, Hou SM, Chen RJ et al. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 26(10), 2552-2563 (2011).
- Farghali H, Kutinová Canová N, Lekić N. Resveratrol and related compounds as antioxidants with an allosteric mechanism of action in epigenetic drug targets. Physiol. Res. 62(1), 1-13 (2013).
- Shakibaei M, Shayan P, Busch F et al. Resveratrol mediated modulation of sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS. One. 7(4), (2012).
- Saunier E, Antonio S, Regazzetti A et al. Resveratrol reverses the Warburg effect by targeting the pyruvate dehydrogenase complex in colon cancer cells. Sci. Rep. 7(1) (2017).