Review Article - Interventional Cardiology (2011) Volume 3, Issue 4
Use of multislice computed tomography angiography in percutaneous coronary intervention
- Corresponding Author:
- Christophe Caussin
Hôpital Marie Lannelongue, 133 Avenue de la Résistance
92350 Le Plessis Robinson, France
Tel: +33 140 948 547
Fax: +33 140 948 549
E-mail: c.caussin@ccml.fr
Abstract
Keywords
aorto-ostial lesion, bifurcations, chronic total occlusion, in-stent restenosis, multislice computed tomography angiography, percutaneous coronary intervention
Coronary artery disease is the leading cause of death and disability in the USA and other Western countries. Coronary angiography (CA) is currently the reference test for coronary artery stenosis assessment, and its use has been steadily increasing over the last decade [1,2]. Although CA is mandatory for percutaneous coronary intervention (PCI), the interpretation of CA in this field has limitations, providing only bidimensional luminal projections, not considering atheroma location, plaque composition and tridimensional structure.
The recent technical developments in multislice computed tomography angiography (MSCT), with ECG retrospective-gated image reconstruction, have elicited great interest in the possibility of accurate noninvasive imaging of the coronary arteries. MSCT seems to respond adequately to the need for a noninvasive and reliable assessment of the coronary artery lesions. Several studies have already shown that the contribution of MSCT has become relatively well established in the diagnosis of coronary disease [3]. The introduction of MSCT provides new information and impacts multiple facets of PCI.
When planning a PCI, MSCT has shown to be of interest in complex lesions. It provides valuable measurements about coronary lesions, which are important predictors of procedural success such as in chronic total occlusions (CTOs), in bifurcations and recently in aortoostial lesion PCI. In multiple areas, tomographic intravascular analysis facilitates decision making by providing data unobtainable from CA.
The aim of this article is to assess the contribution of MSCT in PCI.
Pre-PCI patient selection
Because of its excellent specificity and good sensitivity, MSCT has become one of the routine explorations for coronary artery disease diagnosis.
▪ Diagnosis
The traditional patient evaluation paradigm employs nuclear or echocardiographic stress testing to identify patients who are candidates for PCI by evidencing >10% ischemic area. The sensitivities and specificities for these modalities (86 and 67% for nuclear and 85 and 75% for echocardiographic stress testing, respectively [4]) are considerably less than for 64-slice MSCT (90 and 95%, respectively [5]), suggesting that MSCT is the preferred initial test, especially when ruling out coronary disease.
Moreover, nuclear in particular is problematic in situations where accurate evaluation is most important: 40% of patients with triple vessel disease will have normal (12%) or low-risk single vessel ischemia images (28%) [6]. In patients with left main disease, 15% will have normal (10%) or low-risk (5%) exploration [7]. Stress testing is complementary to MSCT in determining the functional significance of 50–75% of stenoses, in which case significant ischemia would justify PCI, and the absence therefore would suggest medical treatment.
▪ Identification of significant lesions
Multislice computed tomography, by imaging the vessel wall as well as the lumen from a 360° perspective, offers distinct advantages compared with CA despite the superior temporal and spatial resolution of CA (~20 ms and 0.2 mm, respectively) compared with MSCT (75–165 ms and 0.4–0.5 mm, respectively). MSCT can achieve a temporal resolution of 50 ms with some reconstruction methods:
▪ Insufficient sampling error: the 360° perspective provides an infinite number of viewing angles to ensure the stenosis is captured in its narrowest dimension, compared with the insufficient sampling error inherent in the limited number of acquisitions of CA (six to seven for the left, and two to four for the right coronary arteries);
▪ Overlap: overlapping vessels, which frequently complicate CA, are never an issue for CTA; each vessel is tracked independently;
▪ Foreshortening: the least foreshortened view of each vessel is readily determined by available algorithms;
▪ Remodeling and reference areas: accurate calculation of percent diameter stenosis is predicated on identification of appropriate proximal and distal reference areas, a task complicated by the inability of CA to identify areas of positive and negative remodeling. MSCT is ideally suited for this purpose and remodeling index is an easily measurable parameter [8], thereby facilitating the appropriate choice of reference areas. Quantitative MSCT should always be employed; ‘eyeballing’, with its inherent overestimation, is not an acceptable method of analysis, despite being the standard of care for CA;
▪ Minimum lumen area (MLA) calculation: MLA is a far more important physiologic parameter than minimum lumen diameter, and can be reliably derived by tomographic intravascular analysis of cross-sectional images, with correlation with intravascular ultrasound (IVUS) measurements [9,10]. MLA cannot be derived from CA;
▪ Diffuse narrowing: segments with relatively uniform diffuse lumen reduction will be angiographically unapparent on CA since there is no appropriate reference point, and MLA calculations are not available. The absence of a reference area is not problematic for MSCT since the MLA can be determined.
▪ Post-catheterization complementary information
MSCT may be used to resolve questions left unanswered by CA, impacting directly on PCI in situations where complete delineation of coronary or graft anatomy is crucial for therapeutic strategy.
Failure of image acquisition
The most common situations are inability to selectively cannulate a vessel, including anomalous coronary arteries, native coronaries originating from an aneurysmally dilated aortic root, right internal mammary grafts, and vein grafts originating from unanticipated aortic locations. Occasionally, severe pressure damping of the left main or right coronary artery may preclude safe contrast injection. MSCT may provide the necessary information, showing obstructive atheroma.
Requirement for additional information
Even after successful selective CA, there may be unresolved questions for which MSCT is invaluable. These include differentiating ostial disease from coronary spasm unrelieved by intracoronary nitroglycerin, determining the potentially malignant anterior versus the benign posterior course of anomalous coronaries, establishing the relationship of mammary and vein grafts to the sternum to avoid transection during repeat bypass surgery, distinguishing venous bypass aneurysms from pseudoaneurysms, and demonstrating the path and length of CTOs.
Myocardial infarction imaging
imaging Early PCI has improved the prognosis of patients after myocardial infarction (MI). Nevertheless, patency of the epicardial coronary artery after primary PCI does not guarantee reflow at a microvascular level or functional recovery. Knowledge about myocardial perfusion and transmural infarct size after MI has prognostic value and therapeutic consequences. The ability of cardiac magnetic resonance (MR) imaging to help assess both of these parameters is well documented, and MR imaging is often regarded as the clinical standard [11]. Contrast material-enhanced computed tomography can help visualize infarcted myocardium during early and delayed imaging. MSCT allows imaging of early and late myocardial hypoenhancement after reperfused MI, with good correlation with MR imaging, although imaging of delayed hyperenhancement at MSCT has inferior contrast-to-noise ratio. Nevertheless, delayed hyperenhancement at MSCT correlates well with that at MR imaging [12].
Procedure planning
After proving its relevance in the diagnosis of coronary artery disease, MSCT has recently emerged as a useful tool in PCI, especially in treating complex lesions such as CTOs, ostial lesions and bifurcations including those affecting the left main trunk.
▪ Chronic total occlusion
Chronic total occlusions can be found in a third of patients referred for diagnostic invasive CA [13]. They are angiographically defined as an obstruction of a native coronary artery with no luminal continuity and interruption of anterograde flow, with thrombolysis in myocardial infarction grade 0 or 1 and occlusion periods exceeding 3 months.
Chronic total occlusions are a common reason for referral to bypass surgery owing to the relatively high failure rates when attempting a PCI.
Unlike conventional CA, cardiac MSCT provides an accurate assessment of the length and composition of the occluded segment, which are important predictors of procedural success. Preprocedural MSCT guidance therefore has the potential to identify which patients are most likely to benefit from attempted PCI.
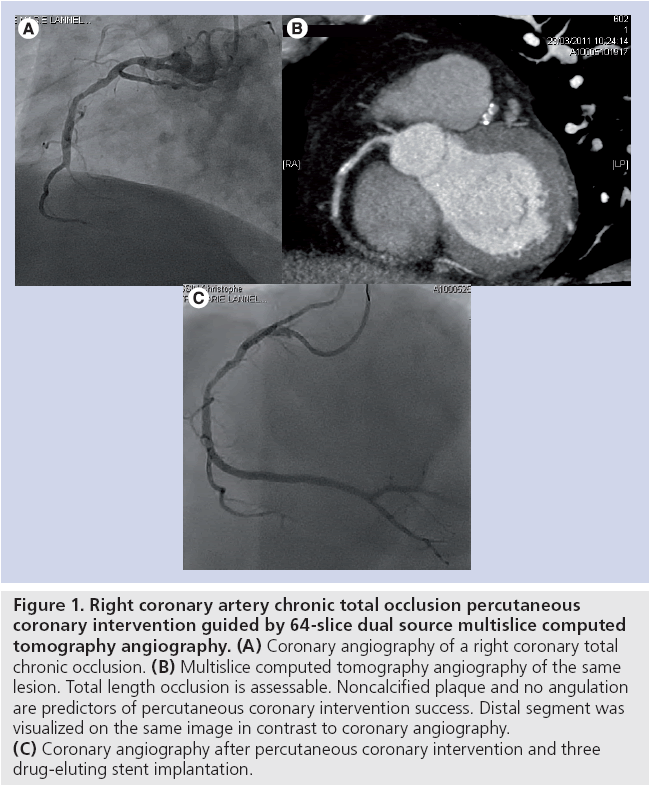
Multislice computed tomography can be utilized to directly guide the procedure in the catheterization laboratory. While segments of the totally occluded vessel may not be visualized by CA, they are always apparent on MSCT, and their visualization will facilitate passage of the guidewire. In particular, attempted opening of flush occlusions may result in fruitless attempts to locate the entrance to the CTO without the guidance provided by MSCT mapping (Figure 1).
Figure 1: Right coronary artery chronic total occlusion percutaneous
coronary intervention guided by 64-slice dual source multislice computed
tomography angiography. (A) Coronary angiography of a right coronary total
chronic occlusion. (B) Multislice computed tomography angiography of the same
lesion. Total length occlusion is assessable. Noncalcified plaque and no angulation
are predictors of percutaneous coronary intervention success. Distal segment was
visualized on the same image in contrast to coronary angiography.
(C) Coronary angiography after percutaneous coronary intervention and three
drug-eluting stent implantation.
Chronic total occlusions appear as a complete lack of contrast opacification of the artery lumen in MSCT images and 3D reconstructions. The distal vessel lumen is often opacified, although less intensely, via filing of the collaterals. In fact, lack of contrast in the distal segment should raise the suspicion of an acute of sub-acute occlusion, which is usually associated with higher success rates of PCI. The occluded segment usually has a different attenuation from the surrounding nonvascular tissue and can be readily identified. Nonetheless, differentiation between total and subtotal occlusions is not reliable with MSCT, in part due to its limited spatial resolution. Longitudinal sections and cross-sectional images of the occluded segment may reveal varying degrees and patterns of calcifications. 3D volume rendering images allow proper visualization of the orientation of the different segments of coronary arteries in space since they can be rotated around any axis. This provides better understanding of the characteristics of the vessel as well as the occluded segment, especially length, tortuosity, angles with side branches, blunt stumps and distal vessel diameter.
Multislice computed tomography is more sensitive in detecting, quantifying and localizing calcification in nonoccluded vessels when compared with CA [14,15]. In occluded vessels, MSCT has been shown to be the best available alternative for characterization of the composition of the plaque (soft, mixed or fibrocalcified) in the missing segment.
Calcifications determined by CA are associated with a lower success rate of CTO PCI. This observation and the increasing utility and availability of MSCT have inspired operators to investigate whether MSCT better than CA at characterizing CTOs and predicting success of PCI. Mollet et al. were the first to point out the value of preprocedural MSCT [15]. Soon et al. explored the association between the degree of MSCT calcification length and the degree of CTO PCI success in 43 CTO lesions with 16-slice MSCT [16]. The degree of transluminal calcification was defined as the area of luminal calcification in relation to the total vessel crosssectional area at the point in the occlusion showing the greatest extent of calcification. A more than 50% transluminal calcification was the only predictor of unsuccesful PCI. Thus, CTOs with >50% transluminal calcification were ten times less likely to be treated successfully. MSCT calcification length in the occluded segment did not show association with PCI success, whereas CTO lesion length showed a weak association. This study confirmed that MSCT angiography is better at quantifying calcification in CTOs than conventional CA and added that the distribution of calcium within the lumen had a great impact on PCI success. By studying 84 CTOs de novo with 64-slice MSCT, Hsu et al. demonstrated that heavy calcification was a strong independent parameter that not only influenced technical success but also procedural success and that a calcification length ratio of >0.5 remains to be the independent negative predictor of both technical and procedural success [17].
To better evaluate the best predictive characteristics of CTO calcification in PCI success, Cho et al. compared calcium volume, calcium concentration, calcium equivalent mass and calcium score, and occlusion length from images acquired with 64-slice MSCT in a group of 64 patients with 72 CTO lesions [18]. All calcium parameters were higher in patients with procedural failure. The percentage cross-sectional calcium area was an independent predictor, confirming results from earlier studies. Additionally, a cutoff value of 53.86% was found to have excellent sensitivity and specificity to predict failure.
The site of calcification along the length of the lesion is also important. In fact, occurrence of calcification at the entry site of the occlusion as opposed to the exit site makes the anterograde approach of recanalization more difficult [14]. This may be a reason for the increasing success rates of wiring the occlusion from the distal cap via collaterals in the retrograde approach [19].
Recently, Ehara et al. studied patients with calcification both on the inside and at both extremities of the occluded site with severe transluminal calcification quantified as high density plaques of >500 Hounsfield unit (HU) [20]. A 64-slice MSCT was performed in 110 patients with the same number of CTOs. Severe calcification was again an independent predictor of wiring success. The other MSCT, derived morphological features that determined CTO PCI success in this study were shrinkage and bending of the target vessel.
Shrinkage in the vessel, identified as abrupt narrowing or severe tapering of the distal portion to less than 1 mm in cross-sectional diameter, is probably an age effect of the CTO. This may explain why the guidewire often tracks outside the vessel wall close to the distal cap, producing a perforation.
Bending, that is, an angle of >45° in the trajectory of the vessel, either the occluded site or proximal vessel, was the most prominent predictor of the three morphological features (the other two being severe calcification and shrinkage). Moreover the presence of binding and the trajectory of the missing segment can be also appreciated by MSCT.
These five studies and the MSCT-derived parameters that showed predictive value for PCI success are summarized in Table 1.
Table 1: Assessment of the predictive value of multislice computed tomography angiography-derived parameters for chronic total occlusion percutaneous coronary intervention.
The 3D nature of MSCT provides more information for vessel anatomy visualization and lesion location, than the invasive 2D angiography. Suboptimal projections with foreshortening, vessel overlap and problems with sizing from conventional CA often do not provide the clear picture required to plan an interventional or surgical procedure on the coronary tree. CTOs occurring in the left main or other ostial locations or in patients with anomalous coronary arteries are better visualized by MSCT and are often better treated by bypass grafting.
Therefore, for planned PCI, preprocedure MSCT of CTOs provides a roadmap. Devices can be selected according to the nature of the occluding plaque, that is, either soft (lipidic) or hard (fibrocalcic). Moreover, the use of such images during the procedure can provide a visual landmark that can be used to steer the guidewire in the proper direction. The utilization of intra procedural MSCT data is a subject of much interest and is currently being explored.
▪ Intraprocedural use of MSCT data
Coronary angiography images can be coupled in the offline MSCT workstation, allowing superimposition of MSCT data. This superimposition allows the operator to visualize the trajectory and the borders of the missing segment, and to localize soft and calcified spots identified from the MSCT images in the invasive counterpart. A specific application for this is in the use in ostial occlusions, which are most challenging on lumenography, and where MSCT overlay might be helpful. Initial software is limited to still frames, and a substantial amount of operator interaction is necessary. However, it has the potential to reduce the fluoroscopy time and contrast that would otherwise be necessary to regularly check the position of the guidewire in relation to the distal vessel. In addition, in the long term this could eventually result in lower procedure time.
Multislice computed tomography reveals the anatomy of CTOs and offers predictive value for the success rate of this high-risk PCI. Its incremental value over CA in this respect allows better risk evaluation that should lead to an informed choice of the best treatment option for the patient.
Ostial lesions
Ostial lesion management is challenging regarding either diagnosis and intervention. Because of their position, the diagnostic angiography catheter crosses the lesion, limiting a precise evaluation of the severity and the exact location of the atheroma, originating or not from the aorta. The complexity of the analysis of such lesions lies in the fact that CA contributes less information in some cases.
Differentiation between aorto-ostial and ostial lesion appears to be warranted to make a proper decision regarding angioplasty [21].
Unlike IVUS, MSCT has shown the ability to detect and classify calcified and noncalcified coronary plaques [7,22,23].
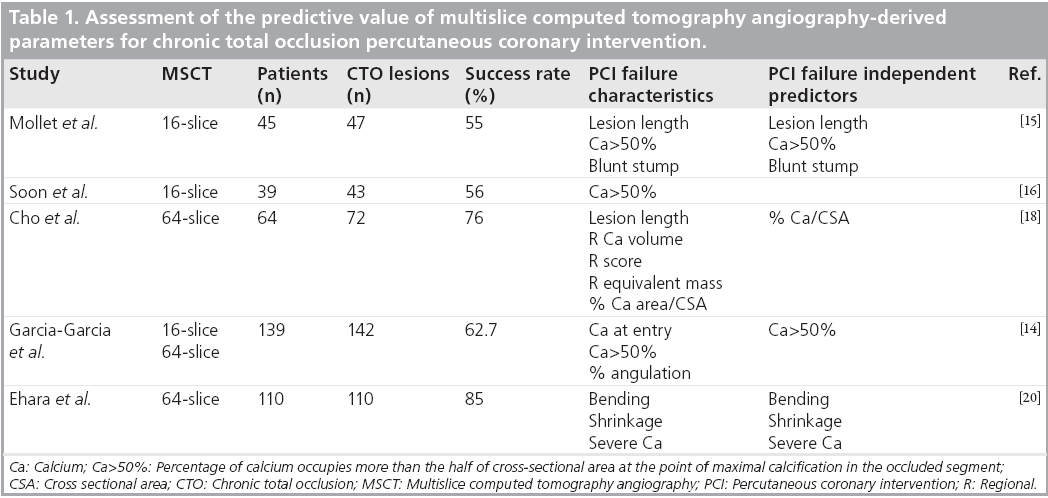
Pesenti-Rossi et al. analyzed 40 aorto-ostial lesions in 38 patients who underwent both MSCT and CA. Two independent observers analyzed MSCT and CA images independently in comparison to an expert consensus analysis [24]. Using MSCT in addition to CA permitted observers to obtain a strong agreement for assessment of calcified lesions (k value 0.75), and a good agreement for aortic plaque location and ideal stent position in aorto-ostial coronary stenosis. In fact, MSCT associated with CA allows for a better assessment of aorto-ostial plaque morphology and was able to identify different types of coronary aorto-ostial stenosis depending on plaque location (aortic or exclusively coronary) and the degree of calcification with a better reproducibility than CA [25,26]. Moreover, the best stent position could be determined with a better reproducibility than CA alone (k value >0.75) (Figure 2).
Figure 2: Example of ostial stent positioning using multislice computed tomography angiography. Sagittal (A) and long axis view (B) of an ostial left main stenosis. Multislice computed tomography provides an accurate 3D analysis. A calcified element is present in the aortic wall but is not involved in the obstruction. Location of the obstructed atheroma is within the coronary artery. However, because of angulation, and to have a sufficient coverage of the stenosis, the stent needs to protrude partially into the aorta (blue modelization).
Percutaneous coronary intervention of aortoostial lesions is associated with lower success and higher complication rates especially instent restenosis, which could be due to stent misplacement. Mavromatis et al. reported that distal stent misplacement in ostial angioplasty may have contributed to increased rates of restenosis [21]. Pesenti-Rossi et al. demonstrated that the use of MSCT by observers provides better visualization of both the angle formed by the junction between the ostial coronary artery and the aortic wall, as well as aortic plaque position, which can decrease the difficulty of stent placement and the possibility of restenosis (Figure 2) [24]. With this information, it is suggested that a reduction of incorrect stent placement using MSCT may improve long-term results.
The use of MSCT with CA has enabled the classification of ‘massive’ or ‘moderate’ calcified lesions. This was useful for selecting patients to undergo rotational atherectomy.
Ostial lesions are therefore a real challenge, especially in terms of PCI, and that is where MSCT has a privileged place in the management of such complex lesions, by refining lesion analysis. However, the use of MSCT can only be strengthened with further studies on a larger scale.
Bifurcation lesions
A bifurcation lesion is known to be technically challenging and is also associated with a high restenosis rate, particularly in true bifurcation lesions (since the beginning of the bare-metal stent era) [27,28].
Bifurcation lesions are well known as a challenging situation for stent deployment owing to their lower procedural success and higher chronic restenosis rate [27,28]. As a result, drug-eluting stents have recently become aggressively used for the treatment of bifurcation lesions owing to their reduced chronic restenosis and target lesion revascularization rate. Various bifurcation stent techniques have been reported. Among these techniques, the crush stent technique described by Colombo et al. has attracted a great deal of attention because it can cover the side-branch ostium with no gap, thereby reducing the side-branch restenosis in comparison to traditional two-stent techniques [29–31]. Recent studies, however, have disclosed limitations regarding the relationship between the degrees of bifurcation angle and stent distortion, incomplete stent apposition and stent expansion in the crush stent technique [32–34]. These findings highlighted the fact that the understanding of the bifurcation geometry and bifurcation angle plays a very important role in selecting the optimal strategies for bifurcation stenting.
Recent MSCT technology provides 3D data sets of the coronary arteries, and information regarding the bifurcation geometry and bifurcation angle can be measured correctly with reproducibility using a 3D image, even if it is measured in arbitrary directions. Kawasaki et al. demonstrated the natural bifurcation geometry in humans while also evaluating the validity of the crush stent technique in the treatment of bifurcation lesions using MSCT [35]. They demonstrated that 3D reconstruction using MSCT has a lower inter-observer variability than CA, thus permitting more accurate measurements of the coronary bifurcation angles, the geometry and bifurcations lesions.
Bifurcation study using MSCT can clarify the 3D structure of natural coronary bifurcation geometries in humans and highlights the limitations of the traditional 2D model analysis. Moreover, information concerning the specific bifurcation geometry may provide useful strategic information for the performance of bifurcation stenting.
Stent follow-up after PCI
The role MSCT remains questionable when monitoring stents after implantation.
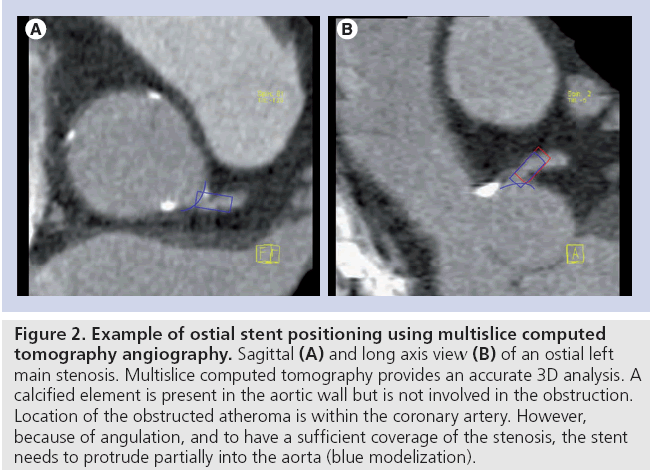
Most patients who have undergone PCI will need diagnostic CA again at some point in time. Recurrent symptoms may be caused by in-stent restenosis or disease progression (Figure 3).
Figure 3: Left circumflex suspected in-stent restenosis in 64-slice dual source multislice computed tomography angiography. Multislice computed tomography image of a 3.5 colbalt-chromium alloy stent (A) in the left circumflex. Stent lumen is filled by black tissue, suspecting in-stent restenosis. (B) Coronary angiography confirms in-stent restenosis. (C) Coronary angiography after a new drug-eluting stent implantation.
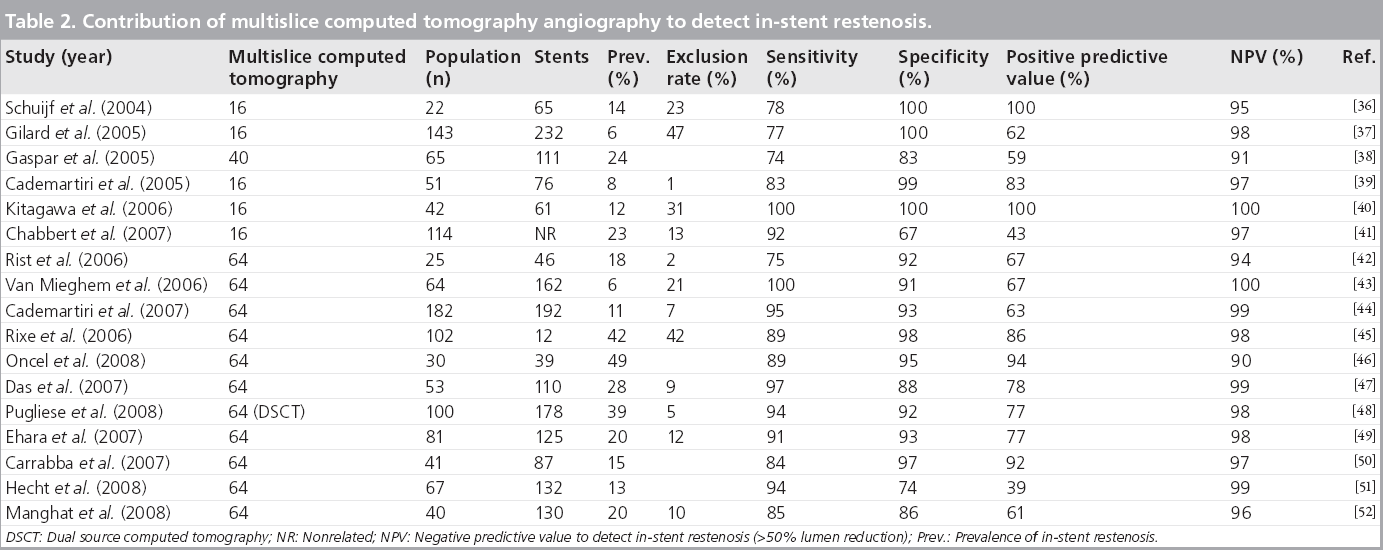
Employing consecutive MSCT technology, numerous studies have been published that investigated the diagnostic performance of MSCT after previous coronary stenting [36–52]. Insufficient image quality required exclusion of up to 46% of the imaged stents. Sensitivity for the detection of restenosis varied between 67 and 100%, with specificity between 74 and 100%.
The heterogeneous results of these studies can at least in part be explained by differences in stents type and size, the relatively small populations studied and the variable prevalence of disease. Different authors reported better image interpretability and diagnostic performance in stents with larger diameters and thinner struts [36,37,40,44–46,48,51]. Stents smaller than 3 mm in diameter are difficult to evaluate. Stents made of tantalum and those containing gold are less interpretable compared with stents made of stainless steel and cobalt alloys [40].
Including a number of recent 64-slice and dual source MSCT studies, the results summarized in Table 2, suggest that the sensitivity of 64-slice MSCT has improved >90%. While the prevalence of in-stent restenosis varies between 6 and 49%, the proportion of complete occlusion is often high (24–59%). Stent occlusion is more easily recognized than in-stent restenosis.
Table 2: Contribution of multislice computed tomography angiography to detect in-stent restenosis.
Because of blooming artefact, a minimal amount of neointimal hyperplasia has to be present for a good visualization by MSCT. This minimal amount was found to be 1 mm in a study comparing MSCT with IVUS [43].
Although the diagnostic performance of current MSCT technology is improving, results are still considered insufficient for positive recommendations of unrestricted use of MSCT in patients with coronary stents [53].
In stent follow-up, stent fracture (another complication), which is hardly recognized by CA, can be easily recognized by MSCT especially through image reconstruction, however, less than severe stent malapposition is beyond the reach of current MSCT technology [54,55].
Conclusion and future perspective
Multislice computed tomography has become a valuable tool for complex lesion PCI by providing interesting anatomical 3D information. Better analysis of the occluded segment, ostial or bifurcation lesion with MSCT probably increases the success rate of PCI by providing data that is unobtainable by CA. However, randomized studies are necessary to validate the use of MSCT for PCI.
Multislice computed tomography images that are fully integrated into the catheterization laboratory monitored and used as references for PCI will probably be one of the next steps in the field.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Diagnosis & coronary lesion identification
▪ The excellent specificity and good sensitivity of multislice computed tomography angiography (MSCT) suggests that it is the preferred first test, especially when ruling out coronary disease.
▪ MSCT, by imaging the vessel wall as well as the lumen from a 360° perspective, offers distinct advantages compared with conventional coronary angiography (CA).
Chronic total occlusion percutaneous coronary intervention
▪ MSCT provides an accurate assessment of the length and composition of the occluded segment, which are important predictors of procedural success.
▪ Chronic total occlusion calcification is the most determinant independent factor for the success of percutaneous coronary intervention (PCI).
▪ MSCT is more sensitive in detecting, quantifying and localizing calcification in occluded vessels when compared with CA.
▪ The use of MSCT images during PCI can provide a visual landmark that can be used to steer the guidewire in the proper direction.
Aorto-ostial lesion PCI
▪ Differentiation between aorto-ostial and ostial lesion appears to be warranted to make a proper decision regarding PCI.
▪ The use of MSCT provides better visualization of both the angle formed by the junction between the ostial coronary artery and the aortic wall, as well as aortic plaque position, which can decrease the difficulty of stent placement and the possibility of restenosis.
Bifurcation lesions
▪ Bifurcation study using MSCT clarifies the 3D structure of coronary bifurcation geometries and suggests the limitations of the traditional 2D model analysis.
▪ Information concerning specific bifurcation geometry may provide useful strategic information for the performance of bifurcation stenting.
Stent follow-up
▪ In-stent restenosis is better detected by CA compared with MSCT.
▪ MSCT 3D reconstructions recognize stent fractures better than CA.
References
Papers of special note have been highlighted as:
▪ of interest
- Thom T, Haase N, Rosamond W et al. Heart disease and stroke statistics – 2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 14(113), e85– e151 (2006).
- Agostoni P, Biondi-Zoccai G, de Benedictis ML et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures. Systematic overview and meta-analysis of randomized trials. J. Am. Coll. Cardiol. 44, 349–56 (2004).
- Hamon M, Biondi-Zoccai G, Malagutti P et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional Invasive coronary angiography. J. Am. Coll. Cardiol. 48, 1896–1910 (2006).
- Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA 280, 913–920 (1998).
- Hecht HS, Roubin G. Usefulness of computed tomographic angiography guided percutaneous coronary intervention. Am. J. Cardiol. 99, 871–875 (2007).
- Lima RSL, Watson DD, Goode AR et al. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J. Am. Coll. Cardiol. 42, 64–70 (2003).
- Berman DS, Kang X, Slomka PJ et al. Underestimation of extent of ischemia by gated spect myocardial perfusion imaging in patients with left brain coronary disease. J. Nucl. Cardiol. 14, 521–528 (2007).
- Achenbach S, Ropers D, Hoffmann U et al. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J. Am. Coll. Cardiol. 43, 842–847 (2004).
- Moselewski F, Ropers D, Pohle K et al. Measurement of crosssectional coronary atherosclerotic plaque and vessel areas by 16-slice multi-detector CT: comparison to IVUS. Am. J. Cardiol. 94, 1294–1297 (2004).
- Caussin C, Larchez C, Ghostine S et al. Comparison of coronary minimal lumen area quantification by sixty-four-slice computed tomography versus intravascular ultrasound for intermediate stenosis. Am. J. Cardiol. 98, 871–876 (2006).
- Beek AM, Kuhl HP, Bondarenko O et al. Delayed contrast-enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. J. Am. Coll. Cardiol. 42(5), 895–901 (2003).
- Nieman K, Shapiro MD, Ferencik M et al. Reperfused myocardial infarction: contrastenhanced 64-section CT in comparaison to MR-imaging. Radiology 247, 49–56 (2008).
- Werner GS, Gitt AK, Zeymer U et al. Chronic total coronary occlusions in patients with stable angina pectoris: impact on therapy and outcome in present day clinical practice. Clin. Res. Cardiol. 98, 435–441 (2009).
- Garcia-Garcia HM, Van Mieghem CA, Gonzalo N et al. Computed tomography in total coronary occlusions (CTTO registry): radiation exposure and predictors of successful percutaneous intervention. EuroIntervention 4, 607–616 (2009).
- Mollet NR, Hoye A, Lemos PA et al. Value of preprocedure multislice computed tomographic coronary angiography to predict the outcome of percutaneous recanalization of chronic total occlusions. Am. J. Cardiol. 95, 240–243 (2005).
- Soon KH, Cox N, Wong A et al. CT coronary angiography predicts the outcome of percutaneous coronary intervention in chronic total occlusion. J. Interv Cardiol. 20, 359–366 (2007).
- Hsu JT, Kyo E, Chu CM, Tsuji T, Watanabe S. Impact of calcification length ratio on the intervention for chronic total occlusions. Int. J. Cardiol. 150(2), 135–141 (2010).
- Cho JR, Kim YJ, Ahn CM et al. Quantification of regional calcium burden in chronic total occlusion by 64-slice multidetector computed tomography and procedural outcomes of percutaneous coronary intervention. Int. J. Cardiol. 145(1), 9–14 (2009).
- Sianos G, Barlis P, Di Mario C et al. European experience with the retrograde approach for the recanalisation of coronary artery chronic total occlusions. A report on behalf of the euroCTO club. EuroIntervention 4, 84–92 (2008).
- Ehara M, Terashima M, Kawai M et al. Impact of multislice computed tomography to estimate difficulty in wire crossing in percutaneous coronary intervention for chronic total occlusion. J. Invasive Cardiol. 21, 575–582 (2009).
- Mavromatis K, Ghazzal Z, Veledar E et al. Comparaison of outcomes of percutaneous coronary intervention of ostial verus nonostial narrowing of the major epicardial coronary arteries. Am. J. Cardiol. 94, 583–587 (2004).
- Kuettner A, Kopp AF, Schroeder S et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in patients with angiographically proven coronary arteries disease. J. Am. Cardiol. 43, 831–839 (2004).
- Gregory SA, Ferencik M, Achenbach S et al. Comparison of sixty-four-slice multidetector computed tomographic coronary angiography to coronary angiography with intravascular ultrasound for the detection of transplant vasculopathy. Am. J. Cardiol. 98, 877–884 (2006).
- Pesenti-Rossi D, Chouli M, Gharbi M et al. Coronary aorto-ostial stenosis analysed by mutislice computed tomography: a new tool for percutaneous coronary intervention? EuroIntervention 6, 717–721 (2011).
- Leber AW, Becker A, Knez A et al. Accuracy of 64-slice computed tomography to classify and quantify plaques volumes in proximal coronary system: a comparative study using intravascular ultrasound. J. Am. Cardiol. 47, 672–677 (2006).
- Caussin C, Ohanessian A, Ghostine S et al. Characterization of vulnerable non-stenotic plaque with 16-slice computed tomography compared with intravascular ultrasound. Am. J. Cardiol. 94, 99–104 (2004).
- Al Suwaidi J, Berger PB, Rihal CS. Immediate and long term outcome of intracoronary stent implantation for true bifurcation lesions. J. Am. Coll. Cardiol. 35, 929–936 (2000).
- Yamashita T, Nishida T, Adamian MG et al. Bifurcation lesions: two stents versus one stent – immediate and follow-up results. J. Am. Coll. Cardiol. 35, 1145–1151 (2000).
- Colombo A, Stankovic G, Orlic D et al. Modified T-stenting technique with crushing for bifurcation lesions: immediate results and 30-day outcome. Catheter Cardiovasc. Interv. 60, 145–151 (2003).
- Colombo A. Bifurcation lesions. Ital. Heart J. 6, 475–488 (2005).
- Ge L, Airoldi F, Iakovou I et al. Clinical and angiographic outcome after implantation of drug-eluting stents in bifurcation lesions with the crush stent technique: importance of final kissing balloon post-dilatation. J. Am. Coll. Cardiol. 46, 613–620 (2005).
- Ormiston JA, Currie E, Webster MW et al. Drug-eluting stents for coronary bifurcations: Insights into the crush technique. Catheter Cardiovasc. Interv. 63, 332–336 (2004).
- Murasato Y. Impact of three-dimensional characteristics of the left main coronary artery bifurcation on outcome of crush stenting. Catheter Cardiovasc. Interv. 69, 248–256 (2007).
- Dzavik V, Kharbanda R, Ivanov J et al. Predictors of long-term outcome after crush stenting of coronary bifurcation lesions: importance of the bifurcation angle. Am. Heart J. 152, 762–769 (2006).
- Kawasaki T, Koga H, Seikawa T et al. The bifurcation study using 64 multislice computed tomography. Catheter Cardiovasc. Interv. 73, 653–658 (2009).
- Schuijf JD, Bax JJ, Jukema JW et al. Feasibility of assessement of coronary stent patency using 16-slice computed tomography. Am. J. Cardiol. 15, 427–430 (2004).
- Gilard M, Cornily JC, Pennec PY et al. Noninvasive assessment of left main coronary stent patency with 16-slice computed tomography. Am. J. Cardiol. 95, 110–112 (2005).
- Gaspar T, Halon DA, Lewis BS et al. Diagnosis of coronary in-stent restenosis with multidetector row spiral computed tomography. J. Am. Coll. Cardiol. 46, 1573–1579 (2005).
- Cademartiri F, Mollet N, Lemos PA et al. Usefulness of multislice computed tomographic coronary angiography to assess in-stent restenosis. Am. J. Cardiol. 96, 799–802 (2005).
- Kitagawa T, Fujii T, Tomohiro Y et al. Noninvasive assessement of coronary stents in patients by 16-slice computed tomography. Int. J. Cardiol. 109, 188–194 (2006).
- Chabbert V, Carrie D, Bennaceur M et al. Evaluation of in-stent restenosis in proximal coronary arteries with mutidetector computed tomography (MDCT). Eur. Radiol. 17, 1452–1463 (2007).
- Rist C, von Ziegler F, Nikolaou K et al. Assessement of coronary artery stent patency and restenosis using 64-slice comuted tomography. Acad. Radiol. 13, 1465–1473 (2006).
- Van Mieghem CA, Cademartiri F, Mollet NR et al. Mutislice spiral computed tomography for the evaluation of stent patency after left main coronary angiography and intravascular ultrasound. Circulation 114, 645–653 (2006).
- Cademartiri F, Schuijf JD, Pugliese F et al. Usefulness of 64-slice multislice computed tomography coronary angiography to assess in stent restenosis. J. Am. Coll. Cardiol. 49, 2204–2210 (2007).
- Rixe J, Achenbach S, Ropers D et al. Assessement of coronary artery stent restenosis by 64-slice muti-detector computed tomography. Eur. Heart J. 27, 2567–2572 (2006).
- Oncel D, Oncel G, Tastan A et al. Evaluation of coronary stent patency and in-stent restenosis with dual source Ct cornary angiography without heart rate control. Am. J. Roentgenol. 191, 56–63 (2008).
- Das KM, El-Menyar AA, Salam AM et al. Contrast-enhaced 64-section coronary multidetector CT angiography verus conventional coronary angiography for stent assessement. Radiology 245, 424–432 (2007).
- Pugliese F, Weustink AC, Van Mieghem C et al. Dual source coronary computed tomography angiography for detecting in-stent restenosis. Heart 94, 848–854 (2008).
- Ehara M, Kawai M, Surmely JF et al. Diagnostic accuracy of coronary in-stent restenosis using 64-slice computed tomography: comparison with invasice coronary angiography. J. Am. Coll. Cardiol. 49, 951–959 (2007).
- Carrabba N, Bamoshmoosh M, Carusi LM et al. Usefulness of 64-slice multidetector computed tomography for detecting drug eluting in-stent restenosis. Am. J. Cardiol. 100, 1754–1758 (2007).
- Hecht HS, Zaric L, Jelnin V et al. Usefulness of 64-detector computed tomographic angiography for diagnosing in-stent restenosis in native coronary arteries. Am. J. Cardiol. 101, 820–824 (2008).
- Manghat N, Van Lingen R, Hewson P et al. Usefulness of 64-detector row computed tomography for evaluation of intracoronary stents in symptomatic patients with suspected in-stent restenosis. Am. J. Cardiol. 101, 1567–1573 (2008).
- Schroeder S, Achenbach S, Bengel F et al. Cardiac computed tomography: indications, applications, limitations, and training requirements. Report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur. Heart J. 29, 531–556 (2008).
- Lim HB, Hur G, Kim SY et al. Coronary stent fracture: detection with 64-section multidetector angiography in patients and in vitro. Radiology 249, 810–819 (2008).
- Aoki A, Tanabe J, Inami T et al. Late multiple stent fractures following deployment of Sirolimus-eluting stents for diffuse right coronary artery stenosis. Int. Heart J. 48, 767–772 (2007).
▪ Meta-analysis of 50,000 patients studying the use of multislice computed tomography angiography (MSCT) in coronary lesion diagnosis.
▪ Interesting review studying the use of MSCT in percutaneous coronary intervention (PCI), and comparing with other imaging techniques such as intravascular ultrasound (IVUS).
▪ Interesting paper analyzing the use of MSCT in chronic total occlusion (CTO) PCI planning.
▪ One of the only prospective studies in this field.
▪ One of the only studies which focuses on anatomical analysis of bifurcation lesions.
▪ European Society of Cardiology recommendation of use of MSCT in coronary disease.