Review Article - Interventional Cardiology (2009) Volume 1, Issue 1
Use of optical coherence tomography in interventional cardiology
- Corresponding Author:
- Peter Barlis
MBBS, MPH, FCSANZ, FESC, FRACP, PhD The Northern Hospital
University of Melbourne, 185 Cooper Street, Epping, Victoria 3076, Australia
Tel: +61 384 058 554
Fax: +61 384 058 405
E-mail: pbarlis@unimelb.edu.au
Abstract
Keywords
atherosclerosis, coronary artery, optical coherence tomography, stent
The use of optical coherence tomography (OCT) within the coronary circulation has been greeted with strong interest amongst cardiologists worldwide. The high resolution afforded by this imaging modality is giving new insights into atherosclerotic plaque and the interactions between the vessel wall and stents following implantation. Such resolution is provided by near-infrared light traveling across sophisticated optics to produce a detailed assessment of the coronary artery. Current technology employing frequency domain (FD)-OCT permits a pullback of 20 mm/s meaning such detail can be acquired in a matter of only a few seconds with no or minimal patient intolerance. This report will review the physical properties of OCT, how this modality differs from conventional intravascular ultrasound (IVUS) and explores the safety and clinical applications of OCT within the coronary circulation.
Optical properties
Optical coherence tomography is an optical analog of ultrasound using light rather than sound to produce an image [1–5]. For OCT imaging, low-coherence, near-infrared light with a wave-length of approximately 1300 nm is used since it minimizes the energy absorption in the light beam caused by protein, water, hemoglobin and lipid. The light waves are reflected by the internal microstructures within biological tissues as a result of their differing optical indices. This technique provides a resolution of 10–20 μm in vivo [6]; this level of detail is tenfold greater than the resolution of IVUS (100–150 μm) [5,7].
As the speed of light is much faster than that of sound, an interferometer is required to measure the backscattered light. The interferometer splits the light source into two ‘arms’ – a reference arm and a sample arm, which is directed into the tissue. The light from both arms is recombined at a detector, which registers the socalled interferogram, the sum of the reference and sample arm fields.
Time domain & frequency domain OCT
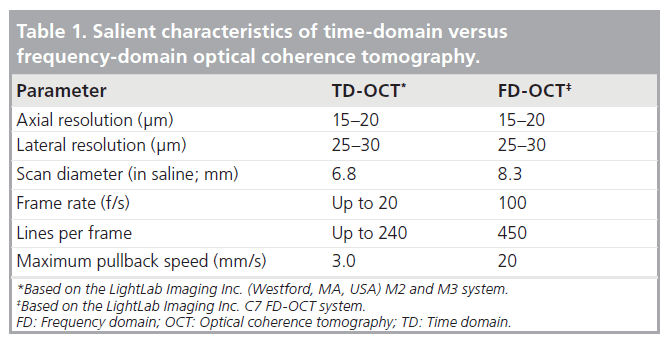
The time-domain (TD)-OCT imaging method on which the first-generation systems (e.g., M2 and M3, LightLab Imaging Inc., Westford, MA, USA) are based relies on a moving mirror to scan each depth position in the image. This mechanical scanning process limits the rate at which images can be acquired. The latest generation of OCT systems employ FD-OCT that enables much faster image acquisition rates and pullback speeds. Such systems (e.g., C7, LightLab Imaging Inc.) incorporate a novel wavelength-swept laser as a light source that has a narrow spectral output [4]. FD-OCT systems can acquire images at line rates at least ten-times faster than TD-OCT systems without loss of image quality. This speed advantage results from the elimination of mechanical scanning of the reference mirror and the signal-to-noise advantages of FD-OCT signal processing [4]. Hence, pullback speeds of approximately 20 mm/s or higher are able to be acquired, ensuring in vivo detailed vessel characterization in a very time-efficient manner. Furthermore, 3D rendering of FD-OCT-acquired pullbacks demonstrates the exciting potential of this technology with imaging of the 3D microstructure of long coronary segments [8]. Table 1 depicts the salient characteristics of TD- versus FD-OCT.
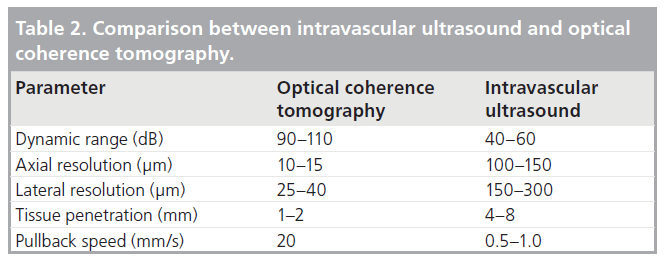
Table 2 lists the differences between OCT and conventional IVUS. The use of light results in a tenfold greater resolution and has seen OCT now be used in clinical studies to assess tissue coverage surrounding coronary stents. In contrast to IVUS imaging however, blood remains an obstacle to OCT imaging and, hence, it must be temporarily cleared during image acquisition. This was initially accomplished by the use of a proximal occlusion balloon catheter and intracoronary flush of lactated ringer’s solution, but can now be accomplished using iso-osmolar viscous contrast flushed via the guiding catheter (see below).
Procedural requirements for image acquisition
As light is unable to penetrate through red cells, blood needs to be cleared from within the coronary artery during image acquisition. This can be accomplished by a number of methods, dependent on the system used.
▪ TD-OCT: balloon occlusion technique
Traditionally, the first-generation TD-OCT systems use a proximal balloon occlusion method whereby a balloon is positioned proximally in the vessel and inflated simultaneously during intracoronary flush. In such systems, the proximal occlusion balloon catheter (Helios, Goodman Co., Japan), an over-the-wire 4.4 Fr catheter (inner diameter: 0.025 inches) is advanced distal to the region of interest using a conventional angioplasty guide wire (0.014 inches) [9]. The guide wire is then replaced by the OCT image wire (0.019 inches maximum diameter), and the occlusion balloon catheter is withdrawn proximal to the segment to be assessed leaving the imaging wire in position. During imaging acquisition, coronary blood flow is removed by continuous flush of Ringer’s lactate solution via the end-hole of the occlusion balloon catheter at a flow rate of 0.5–0.7 ml/s during simultaneous balloon inflation (0.5–0.7 atm) [9].
▪ TD-OCT: nonocclusive technique
Faster OCT pullback speeds have meant that this rather cumbersome technique of blood clearance has been replaced by contrast flush through the guiding catheter during simultaneous image acquisition. Iodixanol (Visipaque™, GE Health Care, Cork, Ireland) is the contrast agent that is preferentially used for flushing during the nonocclusive method [10]. The advantage lies in its higher viscosity relative to other agents, which permits optimal blood clearance for OCT imaging at the given flush volumes through the guiding catheter. This agent has also been shown to have a lower propensity to cause ventricular fibrillation (VF) given its lower osmolality, higher viscosity and higher concentration of sodium and calcium chloride molecules compared with other nonionic media [10–14]. Finally, all such OCT procedures must be performed during therapeutic systemic anticoagulation with liberal use of nitroglycerin to minimize spasm during vessel instrumentation.
▪ FD-OCT: nonocclusive technique
With the current commercial FD-OCT system (C7), the dedicated imaging wire (DragonFly, LightLab Imaging Inc., Westford, MA, USA) is advanced over a standard guidewire via a rapid exchange system. When it is positioned distal to the region of interest, pullback is commenced at 20 mm/s during a short flush of contrast through the guiding catheter [4]. This can be performed either manually or via an automated injector system with flush speed varying depending on the target vessel and its size to achieve adequate blood clearance.
Procedural safety
Optical coherence tomography is an invasive imaging modality and hence has a number of inherent risks. Yamaguchi et al. examined the feasibility of OCT and IVUS imaging in 76 patients [15]. Although transient chest pain and electrocardiographic changes caused by imaging were not considered as part of their study, there were no adverse events reported following both IVUS and OCT, with the latter being performed exclusively using the occlusive method.
More recently, Barlis et al. reported a large multicenter registry of 468 patients having OCT across six European sites [10]. The technique was performed using an occlusion balloon in 256 (54.7%) patients while the nonocclusive approach was used in the remaining 212 (45.3%) cases. The most frequent observation was transient chest pain during OCT image acquisition (47.6% of cases). In all patients this settled following cessation of imaging and was significantly more frequent in patients imaged using the occlusive compared with the nonocclusive technique (69.9 vs 20.8%; p < 0.001). VF occurred in five (1.1%) patients and was linked to deep guide catheter intubation during flushing or the use of the proximal balloon. Several studies have shown that the incidence of VF during coronary angioplasty is approximately 1.5% [16,17], with the rate dropping down to approximately 0.6% for diagnostic procedures [18,19]. In addition to ischemia, other mechanisms have also been identified including reperfusion, electrolyte imbalances, coronary instrumentation, osmolarity and electrolyte composition of contrast agents and intracoronary thrombus [10,20–26].
Clinical applications
The most exciting applications of OCT lie in its ability to clearly define coronary stents and atherosclerotic plaque. With the increased scrutiny placed on stents and, in particular, drugeluting stents (DES), OCT can provide unique information regarding strut apposition and tissue endothelialization, both key factors linked with stent thrombosis [27].
▪ Stent strut apposition
Apposition of stent struts as assessed by OCT has created considerable interest. A number of groups have proposed classification systems of how to define apposed and malapposed struts. Tanigawa et al. divide struts into three categories: first, embedded into the vessel wall; second, protruding into the lumen but still apposed to the vessel wall; and third, malapposed strut with complete separation of the strut from the wall by a distance greater than the strut thickness [9]. Guagliumi et al. propose a four-tier classification with [28]: Totally embedded struts (Type I);
▪ Embedded subintimally without disruption of lumen contour (Type II);
▪ Completely embedded with disruption of lumen contour (Type IIIa);
▪ Partially embedded with extension of strut into lumen (Type IIIb);
▪ Complete strut malapposition (blood able to exist between strut and lumen wall; Type IV).
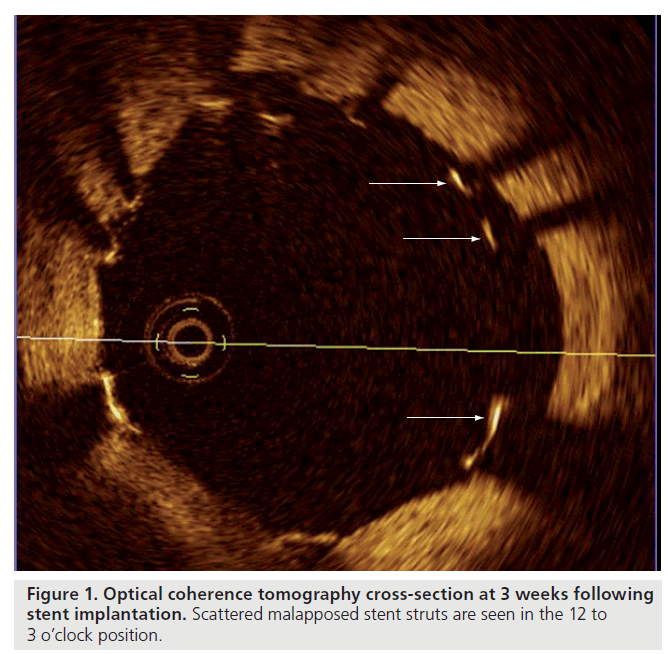
Studies using OCT in the acute setting following stent implantation have demonstrated a high proportion of malapposed struts, even after optimal high pressure postdilatation, with this phenomenon being particularly evident in regions of stent overlap [29]. In an evaluation of OCT findings following stent implantation to complex coronary lesions, Tanigawa et al. examined a total of 6402 struts from 23 patients (25 lesions) and found 9.1 ± 7.4% of all struts in each lesion treated were malapposed [30]. Univariate predictors of malapposition on multilevel logistic regression analysis were: implantation of a sirolimus- eluting stent (SES), presence of overlapping stents, longer stent length and type C lesions. Likely mechanical explanations for malapposition of stent struts include increased strut thickness, closed cell design or acute stent recoil. The latter has been demonstrated in SES to be in the range of 15%, despite the use of high-pressure balloon dilatation [31]. Furthermore, OCT has also been applied to the assessment of strut morphology and apposition in bifurcation lesions treated with dedicated stents (e.g., Tryton bifurcation stent) giving insights into the precise relationship between struts at both the main and side branch, particularly at the carina [32]. While these findings are impressive and helpful for the improvement of future stent designs, the clinical relevance and potential long-term sequalae of malapposed struts as detected by OCT are currently unknown. Figure 1 demonstrates the clarity of detail provided by OCT to visualize malapposed stent struts.
▪ Stent strut tissue coverage
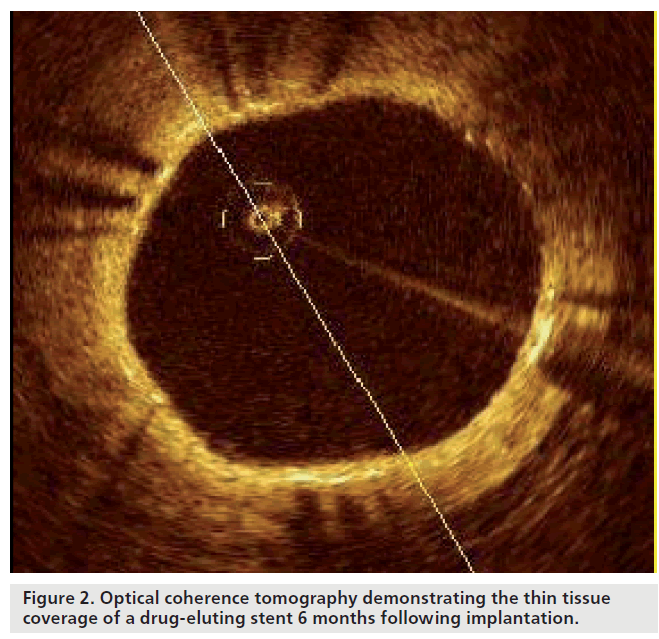
Unlike conventional stents, which develop circumferential coverage with an average thickness of 500 μm or more, well visualized with IVUS and angiography, DES delay and prevent the hyperplastic response so that the average late lumen loss for DES can be lower than 100 μm [33,34], which means this amount of corresponding intimal thickening is difficult to detect by IVUS. Furthermore, although coronary angioscopy is able to visualize strut tissue coverage, this highly specialized technique lacks the ability for quantification. Hence, OCT is an attractive alternative, able to circumvent many of these limitations, and, with its high resolution, can precisely assess the in vivo tissue responses following stent implantation (Figure 2) [35].
Optical coherence tomography can reliably detect early and very thin layers of tissue coverage on stent struts. Several small studies have recently been published highlighting the application of OCT in the detection of stent tissue coverage at follow-up. Importantly, OCT permits the quantification of tissue coverage with high reliability [36]. Matsumoto et al. studied 34 patients 6 months following SES implantation [37]. The mean neointima thickness was 52.5 microns, and the prevalence of struts covered by thin neointima undetectable by IVUS was 64%. The average rate of neointima-covered struts in an individual SES was 89%. Nine SES (16%) showed full coverage by neointima, whereas the remaining stents had partially uncovered struts. Similarly, Takano et al. studied 21 patients (4516 struts) 3 months following SES implantation [38]. Rates of exposed struts and exposed struts with malapposition were 15 and 6%, respectively. These were more frequent in patients with acute coronary syndrome (ACS) than in those with non-ACS (18 vs 13%; p < 0.001 and 8 vs 5%; p < 0.005, respectively). The same group have recently reported 2‑year follow-up OCT findings with the thickness of neointimal tissue at 2 years being greater than that at 3 months (71 ± 93 μm vs 29 ± 41 μm, respectively; p < 0.001) [39]. Frequency of uncovered struts was found to be lower in the 2‑year group compared with the 3‑month group (5 vs 15%, respectively; p < 0.001) and, by contrast, prevalence of patients with uncovered struts did not differ between the 3‑month and the 2‑year group (95 vs 81%, respectively), highlighting that exposed struts continued to persist at long‑term follow-up.
Chen et al. used OCT to image SES and bare‑metal stents (BMS) at different time points following implantation [40]. Of the ten SES and 13 BMS imaged, the authors identified a significantly higher number of incompletely apposed and uncovered stent struts in patients receiving SES compared with BMS. The results of these small observational studies are compatible with evidence from animal and human post-mortem series showing that DES cause impairment in arterial healing with incomplete re-endothelialization and persistence of fibrin possibly triggering late stent thrombosis [27,41,42].
In a small, randomized trial of a polymer versus polymer-free DES, OCT was performed at 3-month follow-up and found a significantly large percentage of malapposed, uncovered and protruding struts in the SES (Cypher®, Cordis, Johnson & Johnson) compared with a nonpolymer SES (Yukon®, Translumina, Hechingen, Germany) [43]. In this study, Moore et al. hypothesized that the profile and thickness of the polymer-based SES and its permanent polymer may have a bearing on these findings and may potentially influence the long-term risk of stent thrombosis with the polymer being a contributing factor to stent failure [43]. Longer term follow-up with serial OCT stent interrogations may help address such issues definitively.
Plaque assessment
Several imaging modalities have been used to assess and identify vulnerable plaque (VP), including coronary angioscopy, IVUS and magnetic resonance imaging. Recently, there has been significant interest in the field of VP detection using OCT [44–52]. Several morphologic features described in autopsy series are of particular interest in these VPs. These include the presence of a thin fibrous cap, a necrotic lipid core [11,12] and the accumulation of macrophages [13]. However, at this point in time, no longitudinal studies have been performed to assess the prognostic value of OCT-derived plaque characterization.
Optical coherence tomography is highly sensitive and specific for the characterization of plaques when compared with histological examination [53–55]. Yabushita et al. performed an in vitro study of more than 300 human atherosclerotic artery segments [56]. When compared with histological examination, OCT had a sensitivity and specificity, respectively, of 71–79% and 97–98% for fibrous plaques, 95–96% and 97% for fibrocalcific plaques, and 90–94.5% and 90–92% for lipid-rich plaques. Furthermore, the inter- and intra-observer variability of OCT measurements were high (k values of 0.88 and 0.91, respectively) [55]. In vitro comparisons of OCT with IVUS have demonstrated superior delineation by OCT of structural details such as thin caps, lipid pools or tissue proliferation [57]. An in vitro comparison of OCT, integrated backscatter IVUS (similar methodology to virtual histology [VH]-IVUS) and conventional IVUS found that OCT had the best potential for tissue characterization of coronary plaques, with higher sensitivity and specificity compared with the other imaging modalities [58].
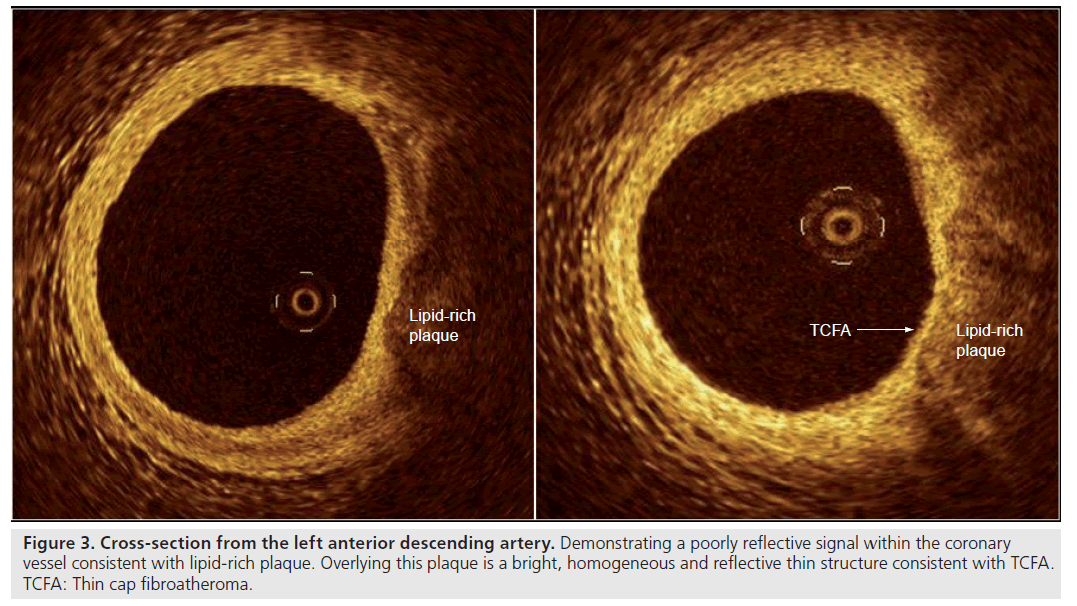
As a result of its high axial resolution, there is no doubt that OCT is the in vivo gold standard for identifying and measuring the thickness of the fibrous cap (Figure 3); an in vivo study found a significant difference in minimal cap thickness between acute myocardial infarction (AMI) and stable angina patients, with median (interquartile range) values of 47.0 μm (25.3–184.3 μm) and 102.6 μm (22.0–291.1 μm) respectively (p = 0.02) [59]. On top of its reliability as a tool to measure the thickness of the cap in vivo, recent post-mortem and in vivo studies have shown that OCT is capable of evaluating the macrophage content of infiltrated fibrous caps [60,61].
Recently, Sawada et al. studied 56 patients with angina (126 plaques) using both VH-IVUS and OCT [62]. A total of 61 plaques were diagnosed as IVUS-derived thin cap fibroatheroma (TCFA) and 36 plaques as OCT-derived TCFA. A total of 28 plaques were diagnosed as definite TCFA indicating that neither modality was able to definitively localize TCFA; however, using both imaging modalities resulted in a greater pick-up rate. This study was limited, however, by the lack of definitive histological confirmation (namely as it was an in vivo analysis) and only used IVUS to localize TCFA.
Kubo et al. used OCT, together with IVUS and angioscopy, to assess plaque characteristics in 30 patients presenting with AMI [44]. The imaging devices were consecutively used following initial mechanical thrombectomy and found the incidence of plaque rupture by OCT to be 73%, which is significantly higher than that detected by both angioscopy (47%; p = 0.035) and IVUS (40%; p = 0.009). The incidence of TCFA was 83% in this patient population and only OCT was able to estimate the fibrous cap thickness (mean: 49 ± 21μm). Furthermore, intracoronary thrombus was observed in all cases by OCT and angioscopy, but was identified in only 33% of patients by IVUS (p < 0.001).
Recently, Gonzalo et al. studied 30 patients with 103 bifurcations using VH-IVUS and OCT [63]. Of the 103 lesions, 17.4% were found to contain TCFA with the overall percentage of necrotic core decreasing from the proximal to distal segments of the bifurcation (16.8 vs 13.5%, respectively; p = 0.01). Cap thickness conversely increased (130 ± 105 μm vs 151 ± 68 μm for proximal and distal rim, respectively; p = 0.05) highlighting the predilection of the proximal segment of the bifurcation to contain thin fibrous cap and a greater proportion of necrotic core. Such data may help provide clearer insights as to why such lesions have a greater complication rate compared with nonbifurcation disease.
Limitations of OCT
Faster pullback speeds now used with FD-OCT systems have signif icantly simplif ied the procedural requirements during imaging. Nevertheless, the need for a blood-free environment requires transient clearance of blood from the vessel and this still adds an element of complexity when compared with IVUS. The presence or absence of tissue strut coverage is clearly as defined by the resolution of OCT, and struts with no visible tissue could have had a thin covering of tissue (<10 μm), although at this level the biological protection of the neointima has been debated [64].
Tissue penetration using OCT is limited to approximately 1.5–2 mm, meaning that regions around the external elastic lamina are more often not visible, thus arterial remodeling is difficult to determine. Nevertheless, much of the interest in interventional cardiology lies in the interaction between the lumen and vessel wall and, therefore, OCT remains particularly effective at characterizing this.
Finally, OCT results in an abundance of data that require off-line image interpretation. At present, this process remains quite cumbersome although there is emerging evidence that automation of this process may significantly reduce the analysis time while maintaining excellent inter- and intra-observer reproducibility [36,65].
Future perspective
The ability of OCT to provide high-resolution imaging in vivo is the most significant concept circumventing the limitations of other imaging modalities such as angiography and IVUS. The identification of vulnerable plaque and the examination of tissue coverage following stent implantation have meant OCT has a clear role to play in future research looking at atherosclerosis and in providing a possible explanation to long-term stent thrombosis. With even faster pullback speeds and simplification of the procedural requirements, as with the current FD-OCT system, OCT will become more accessible to a greater number of centers and operators worldwide. Such advances will also see OCT consistently applied as a multivessel diagnostic modality, able to provide a truly representative assessment of the entire coronary tree while giving never before seen in vivo detail of within the coronary artery.
Executive summary
Optical properties
▪ Optical coherence tomography (OCT) uses near-infrared light with a wavelength of 1310 nm.
Time- versus Fourier-domain OCT
▪ The optical properties of OCT determine the scan length and pullback speed during image acquisition with Fourier-domain systems allowing a very fast 20 mm/s pullback speed.
Procedural requirements
▪ Image acquisition needs to be performed in a blood-free environment. This is now accomplished by flushing of contrast within the coronary artery.
Clinical applications
▪ OCT is well placed to characterize atherosclerotic plaque and also to assess stent strut apposition and tissue coverage in great detail.
Limitations
▪ OCT is an invasive technique. Tissue penetration is limited to up to 2 mm meaning that the external elastic lamina is generally not visualized.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Regar E, Serruys PW: Optical coherence tomography in cardiovascular research. Informa Healthcare, London, UK (2007).
- Huang D, Swanson EA, Lin CP et al.: Optical coherence tomography. Science 254(5035), 1178–1181 (1991).
- Barlis P, Di Mario C, van Beusekom H, Gonzalo N, Regar E: Novelties in cardiac imaging – optical coherence tomography (OCT). EuroIntervention, 4(Suppl. C), C22–C26 (2008).
- Barlis P, Schmitt JM: Current and future developments in intracoronary optical coherence tomography imaging. EuroIntervention 4(4), 529–533 (2009).
- Barlis P, van Soest G, Serruys PW, Regar E: Intracoronary optical coherence tomography and the evaluation of stents. Expert Rev. Med. Devices 6(2), 157–167 (2009).
- Brezinski ME, Tearney GJ, Bouma BE et al.: Imaging of coronary artery microstructure (in vitro) with optical coherence tomography. Am. J. Cardiol. 77(1), 92–93 (1996).
- Regar E, Schaar JA, Mont E, Virmani R, Serruys PW: Optical coherence tomography. Cardiovasc. Radiat. Med. 4(4), 198–204 (2003).
- Tearney GJ, Waxman S, Shishkov M et al.: Three-dimensional coronary artery microscopy by intracoronary optical frequency domain imaging. JACC Cardiovasc. Imaging 1(6), 752–761 (2008).
- Tanigawa J, Barlis P, Di Mario C: Intravascular optical coherence tomography: optimisation of image acquisition and quantitative assessment of stent strut apposition. EuroIntervention 3, 128–136 (2007).
- Barlis P, Gonzalo N, Di Mario C et al. A multicentre evaluation of the safety of intracoronary optical coherence tomography. EuroIntervention 5(1), 90–95 (2009).
- Baath L, Almen T: Reducing the risk of ventricular fibrillation by adding sodium to ionic and non-ionic contrast media with low iodine concentration. Coronary perfusion of the isolated rabbit heart with meglumine diatrizoate or iopentol at 140 mg I/ml and 0–154 mmol Na+/l. Acta Radiol. 30(2), 207–212 (1989).
- Hayakawa K, Yamashita K: Low-osmolality contrast media-induced ventricular fibrillation. Invest. Radiol. 24(4), 298–301 (1989).
- Morris TW, Ventura J: Incidence of fibrillation with dilute contrast media for intra-arterial coronary digital subtraction angiography. Invest. Radiol. 21(5), 416–418 (1986).
- Chai CM, Karlsson JO, Almen T: Incidence of ventricular fibrillation during left coronary arteriography in pigs: comparison of a solution of the nonionic dimer iodixanol with solutions of five different nonionic monomers. Acta Radiol. 49(2), 150–156 (2008).
- Yamaguchi T, Terashima M, Akasaka T et al.: Safety and feasibility of an intravascular optical coherence tomography image wire system in the clinical setting. Am. J. Cardiol. 101(5), 562–567 (2008).
- Bredlau CE, Roubin GS, Leimgruber PP et al.: In-hospital morbidity and mortality in patients undergoing elective coronary angioplasty. Circulation, 72(5), 1044–1052 (1985).
- Dorros G, Cowley MJ, Simpson J et al.: Percutaneous transluminal coronary angioplasty: report of complications from the National Heart, Lung, and Blood Institute PTCA Registry. Circulation 67(4), 723–730 (1983).
- Davis K, Kennedy JW, Kemp HG Jr et al.: Complications of coronary arteriography from the Collaborative Study of Coronary Artery Surgery (CASS). Circulation 59(6), 1105–1112 (1979).
- Nishimura RA, Holmes DR Jr, McFarland TM, Smith HC, Bove AA: Ventricular arrhythmias during coronary angiography in patients with angina pectoris or chest pain syndromes. Am. J. Cardiol. 53(11), 1496–1499 (1984).
- Goldstein JA, Butterfield MC, Ohnishi Y, Shelton TJ, Corr PB: Arrhythmogenic influence of intracoronary thrombosis during acute myocardial ischemia. Circulation 90(1), 139–147 (1994).
- Gorenek B: Tachyarrhythmias in percutaneous coronary interventions. J. Electrocardio. 39(4), 412.e1–412.e5 (2006).
- Huang JL, Ting CT, Chen YT, Chen SA: Mechanisms of ventricular fibrillation during coronary angioplasty: increased incidence for the small orifice caliber of the right coronary artery. Int. J. Cardiol. 82(3), 221–228 (2002).
- Missri J, Jeresaty RM. Ventricular fibrillation during coronary angiography: reduced incidence with nonionic contrast media. Cathet. Cardiovasc. Diagn. 19(1), 4–7 (1990).
- Pedersen HK, Jacobsen EA, Mortensen E, Refsum H: Contrast-medium-induced ventricular fibrillation: arrhythmogenic mechanisms and the role of antiarrhythmic drugs in dogs. Acad. Radiol. 2(12), 1082–1088 (1995).
- Quigley PJ, Maurer BJ: Ventricular fibrillation during coronary angiography: association with potassium-containing glyceryl trinitrate. Am. J. Cardiol. 56(1), 191 (1985).
- Rudoff J, Phillips L: High-osmolality and low-osmolality contrast agents. N. Engl. J. Med. 327(3), 203–204 (1992).
- Joner M, Finn AV, Farb A et al.: Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Guagliumi G, Sirbu V. Optical coherence tomography: high resolution intravascular imaging to evaluate vascular healing after coronary stenting. Catheter Cardiovasc. Interv. 72(2), 237–247 (2008).
- Tanigawa J, Barlis P, Dimopoulos K, Di Mario C: Optical coherence tomography to assess malapposition in overlapping drug-eluting stents. EuroIntervention 3(13), 580–583 (2008).
- Tanigawa J, Barlis P, Dimopoulos K et al.: The influence of strut thickness and cell design on immediate apposition of drugeluting stents assessed by optical coherence tomography. Int. J. Cardiol. 134(2), 180–188 (2009).
- Regar E, Schaar J, Serruys PW: Images in cardiology. Acute recoil in sirolimus eluting stent: real time, in vivo assessment with optical coherence tomography. Heart 92(1), 123 (2006).
- Ferrante G, Kaplan AV, Di Mario C: Assessment with optical coherence tomography of a new strategy for bifurcational lesion treatment: the Tryton Side-Branch Stent™. Catheter Cardiovasc. Interv. 73(1), 69–72 (2009).
- Regar E, Schaar J, van der Giessen W, van der Steen A, Serruys P: Real-time, in vivo optical coherence tomography of human coronary arteries using a dedicated imaging wire. Am. J. Cardiol. 90(Suppl. 6A), 129H (2002).
- Di Mario C, Barlis P: Optical coherence tomography: a new tool to detect tissue coverage in drug-eluting stents. JACC Cardiovasc. Interv. 1(2), 174–175 (2008).
- Regar E, Ong A, Mc Fadden E et al.: Long-term follow-up of drug-eluting stents (DES) – optical coherence tomography (OCT) findings. Eur. Heart J. P4047 (abstract) (2005).
- Tanimoto S, Rodriguez-Granillo G, Barlis P et al.: A novel approach for quantitative analysis of intracoronary optical coherence tomography: high inter-observer agreement with computer-assisted contour detection. Catheter Cardiovasc. Interv. 72(2), 228–235 (2008).
- Matsumoto D, Shite J, Shinke T et al.: Neointimal coverage of sirolimus-eluting stents at 6‑month follow-up: evaluated by optical coherence tomography. Eur. Heart J. 28(8), 961–967 (2007).
- Takano M, Inami S, Jang IK et al.: Evaluation by optical coherence tomography of neointimal coverage of sirolimus-eluting stent three months after implantation. Am. J. Cardiol. 99(8), 1033–1038 (2007).
- Takano M, Yamamoto M, Inami S et al.: Long-term follow-up evaluation after sirolimus-eluting stent implantation by optical coherence tomography: do uncovered struts persist? J. Am. Coll. Cardiol. 51(9), 968–969 (2008).
- Chen BX, Ma FY, Luo W et al.: Neointimal coverage of bare-metal and sirolimus-eluting stents evaluated with optical coherence tomography. Heart 94(5), 566–570 (2008).
- Finn AV, Joner M, Nakazawa G et al.: Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115(18), 2435–2441 (2007).
- Finn AV, Nakazawa G, Joner M et al.: Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler. Thromb. Vasc. Biol. 27(7), 1500–1510 (2007).
- Moore P, Barlis P, Spiro J et al.: A randomized optical coherence tomography study of coronary stent strut coverage and luminal protrusion with rapamycin-eluting stents. JACC Cardiovasc. Interv. 2(5), 437–444 (2009).
- Kubo T, Imanishi T, Takarada S et al.: Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 50(10), 933–939 (2007).
- Chia S, Christopher Raffel O, Takano M et al.: In vivo comparison of coronary plaque characteristics using optical coherence tomography in women vs. men with acute coronary syndrome. Coron. Artery Dis. 18(6), 423–427 (2007).
- Tearney GJ, Jang IK, Bouma BE: Optical coherence tomography for imaging the vulnerable plaque. J. Biomed. Opt. 11(2), 021002 (2006).
- Giattina SD, Courtney BK, Herz PR et al.: Assessment of coronary plaque collagen with polarization sensitive optical coherence tomography (PS-OCT). Int. J. Cardiol. 107(3), 400–409 (2006).
- Jang IK, Tearney GJ, MacNeill B et al.: In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 111(12), 1551–1555 (2005).
- Tearney GJ, Yabushita H, Houser SL et al.: Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 107(1), 113–119 (2003).
- Regar E, Schaar JA, Mont E, Virmani R, Serruys PW: Optical coherence tomography. Cardiovasc. Radiat. Med. 4(4), 198–204 (2003).
- Jang IK, Bouma BE, Kang DH et al.: Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J. Am. Coll. Cardiol. 39(4), 604–609 (2002).
- Barlis P, Serruys PW, Gonzalo N et al.: Assessment of culprit and remote coronary narrowings using optical coherence tomography with long-term outcomes. Am. J. Cardiol. 102(4), 391–395 (2008).
- Jang IK, Bouma BE, Kang DH et al.: Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J. Am. Coll. Cardiol. 39(4), 604–609 (2002).
- Patwari P, Weissman NJ, Boppart SA et al.: Assessment of coronary plaque with optical coherence tomography and high-frequency ultrasound. Am. J. Cardiol. 85(5), 641–644 (2000).
- Yabushita H, Bouma BE, Houser SL et al.: Characterization of human atherosclerosis by optical coherence tomography. Circulation 106(13), 1640–1645 (2002).
- Yabushita H, Bouma BE, Houser SL et al.: Characterization of human atherosclerosis by optical coherence tomography. Circulation 106(13), 1640–1645 (2002).
- Brezinski ME, Tearney GJ, Weissman NJ et al.: Assessing atherosclerotic plaque morphology: comparison of optical coherence tomography and high frequency intravascular ultrasound. Heart 77(5), 397–403 (1997).
- Kawasaki M, Bouma BE, Bressner J et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J. Am. Coll. Cardiol. 48(1), 81–88 (2006).
- Jang IK, Tearney GJ, MacNeill B et al.: In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 111(12), 1551–1555 (2005).
- Tearney GJ, Yabushita H, Houser SL et al.: Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 107(1), 113–119 (2003).
- MacNeill BD, Jang IK, Bouma BE et al.: Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J. Am. Coll. Cardiol. 44(5), 972–979 (2004).
- Sawada T, Shite J, Garcia-Garcia HM et al.: Feasibility of combined use of intravascular ultrasound radiofrequency data analysis and optical coherence tomography for detecting thin-cap fibroatheroma. Eur. Heart J. 29(9), 1136–1146 (2008).
- Gonzalo N, Garcia-Garcia HM, Regar E et al.: In vivo assessment of high-risk coronary plaques at bifurcations with combined intravascular ultrasound and optical coherence tomography. JACC Cardiovasc. Imaging 2(4), 473–482 (2009).
- Calladine D, Tanner V: Optical coherence tomography of the effects of stromal hydration on clear corneal incision architecture. J. Cataract Refract. Surg. 35(8), 1367–1371 (2009).
- Gonzalo N, Garcia-Garcia HM, Serruys PW et al.: Reproducibility of quantitative optical coherence tomography for stent analysis. EuroIntervention 5(2), 224–232 (2009).
▪▪ The first large study to examine the procedural safety of intracoronary optical coherence tomography (OCT).
▪ Provides postmortem evidence of a link between strut endothelialization and stent thrombosis.
▪ Concise overview of the utility of OCT in examining stent struts.
▪ Demonstrates one of the important applications of OCT, namely in the evaluation of stent strut apposition.
▪ Interesting study comparing OCT and intravascular ultrasound in the examination of atherosclerotic plaque.