Mini Review - Interventional Cardiology (2023) Volume 15, Issue 3
Use of radiofrequency wire and steerable sheath system to streamline workflow for valve-in-valve transcatheter mitral valve replacement
- Corresponding Author:
- Augustin DeLago
Department of Cardiology, Albany Medical Center Hospital, Albany, USA
E-mail: roto11257@aol.com
Received date: 25-Apr-2023, Manuscript No. FMIC-23-97019; Editor assigned: 27-Apr-2023, PreQC No. FMIC-23-97019 (PQ); Reviewed date: 11-May-2023, QC No. FMIC-23-97019; Revised date: 18-May-2023, Manuscript No. FMIC-23-97019 (R); Published date: 26-May-2023, DOI: 10.37532/1755- 5310.2023.15(3).715
Abstract
Valve-in-valve (ViV) transcatheter mitral valve replacement (TMVR) has emerged as a safer alternative for patients with a degenerated bioprosthesis at high-risk for repeat surgical mitral valve replacement. Precise transseptal puncture (TSP) for ViV-TMVR procedures is critical to ensure access to the mitral valve through the left atrium (LA) for optimal device delivery. The VersaCross steerable system, comprised of a RF transseptal wire and steerable sheath, uses radiofrequency (RF) energy to facilitate controlled TSP, and allows repositioning to optimize the puncture site without needle or guidewire exchanges. This, both, streamlines workflows and reduces potential risks of injury or introducing air. This technique paper aims to describe a more streamlined workflow which can improve safety and access for ViV-TMVR procedures
Keywords
Radiofrequency wire; Transseptal puncture; Mitral Valve-in Valve (ViV); Transcatheter Mitral Valve Replacement (TMVR)
Abbreviations
CT: Computed Tomography; LA: Left Atrium; LV: Left Ventricle; LVOT: Left Ventricular Outflow Tract; RF: Radiofrequency; SVC: Superior Vena Cava; TEE: Transesophageal Echocardiography; TAVR: Transcatheter Aortic Valve Replacement; TMVR: Transcatheter Mitral Valve Replacement; TSP: Transseptal puncture; ViV: Valve-in-valve
Introduction
The current gold standard for the treatment of mitral valve disease has been surgical mitral valve replacement when transcatheter mitral valve repair is not indicated [1]. Degeneration of bioprosthetic valves over time, such as through wear and tear or prosthetic valve endocarditis, can limit long-term success [2]. Formation of pannus, thrombus, calcification, or valvular regurgitation can result in mitral valve stenosis and mitral valve degeneration [3]. Repeat surgical procedures carry an increased risk of mortality and complications [4]. Valve-in-Valve (ViV) Transcatheter Mitral Valve Replacement (TMVR), whereby a transcatheter heart valve is used for transcatheter mitral valve replacement, has recently emerged as a safer alternative for patients with a degenerated bioprosthesis at high-risk for repeat surgical mitral valve replacement, with a lower risk of mortality of 5.4% at 30 days and 16.7% at 1 year, and greater procedural success rate of 96.8%, when compared to other TMVR approaches [5,6]. This is typically performed using a transfemoral transseptal approach, wherein transseptal location is critical to ensure that appropriate height can be achieved to access the mitral valve through the Left Atrium (LA). Several tools can be used to optimize Transseptal Puncture (TSP) for ViV procedures. Steerable sheaths can be used to optimize the precise needle position on the septum. Radiofrequency (RF) needles require minimal force for puncture, thereby preventing slippage from the intended target location on the atrial septum. Recent addition of the VersaCross system (Baylis Medical, Montreal, Canada), which uses RF wire to puncture the septum, has also eliminated the need for a stiff exchange wire, allowing direct exchange for the therapy delivery system, thereby offering improved procedural efficiency. This paper describes the tools and technique used at our facility to optimize success and efficiency of Left Ventricular (LV) access for mitral ViV procedures.
Procedural Technique
Imaging
Pre-procedural Computed Tomography (CT) TMVR is used to assess the potential risk for iatrogenic obstruction Left Ventricular Outflow Tract (LVOT) obstruction. Transesophageal Echocardiography (TEE) is used to identify the mechanism for bioprosthetic failure and assess interatrial septal anatomy. If the septum cannot be identified on TEE, then transseptal ViV-TMVR is ruled out. The use of TEE can increase procedure safety and success in patients with a thickened or hypertrophic atrial septum [7]. Furthermore, fluoroscopy is utilized in these procedures to help facilitate transseptal access to LA.
Vascular access and Transseptal puncture
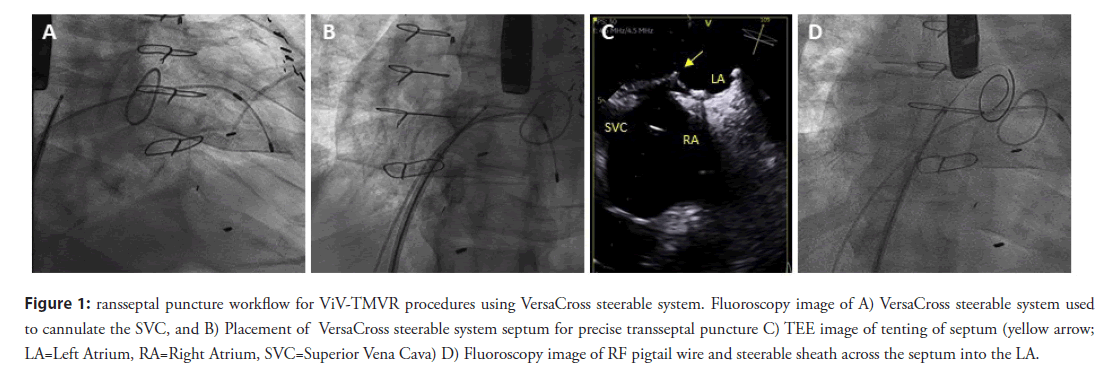
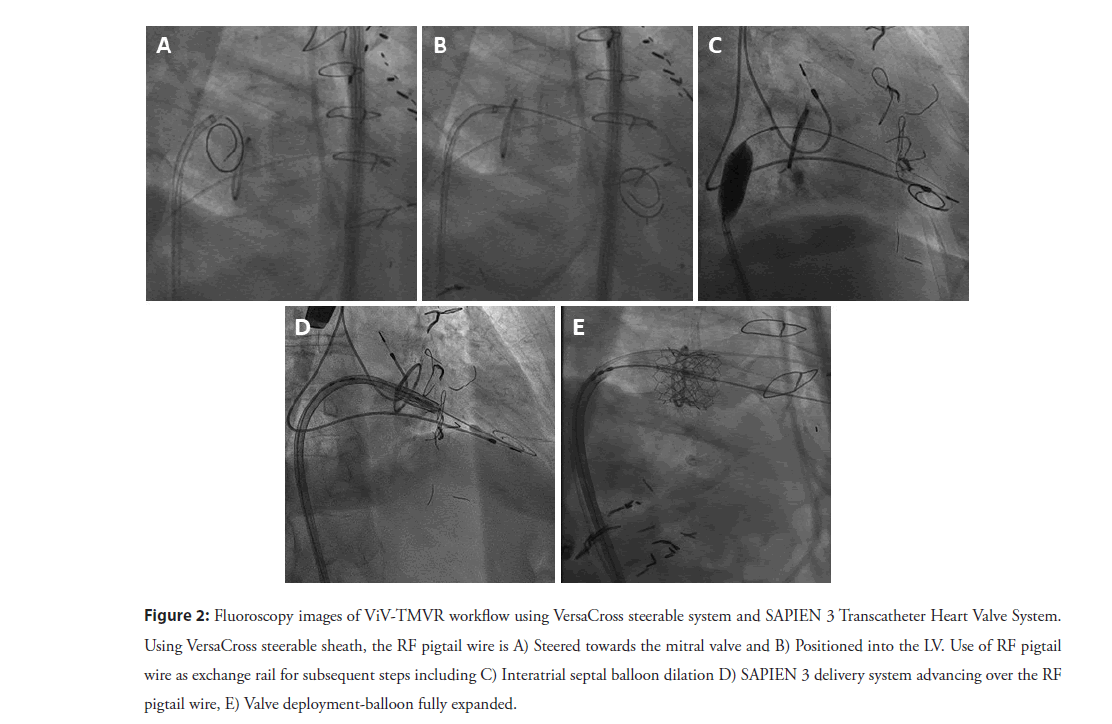
After obtaining vascular access with a 14F Edwards eSheath (Edwards LifeSciences Corp., Irvine, CA, USA), a 0.035” VersaCross RF pigtail wire, the dedicated steerable sheath (VersaCross Steerable Sheath, Baylis Medical) is advanced towards the Superior Vena Cava (SVC) under fluoroscopic guidance (Figure 1A). The pigtail wire can be either pulled back or kept exposed during dropdown to the fossa ovalis. TEE measurements are used to determine height above the mitral annulus and determine optimal puncture location. Typically, a mid-septum location is targeted and adjusted based on patient anatomy to provide sufficient space of 4 mm longitudinal distance, perpendicular to the line of coaptation of the mitral valve for subsequent device delivery (Figure 1B) [8]. Unlike the standard needle-based transseptal workflow, if repositioning of the transseptal assembly is needed to optimize location on the atrial septum, the sheath and wire assembly is pulled back up to the SVC without any device removal or re-wiring. Minimal tenting and forward force are needed to confirm location of the RF wire tip on TEE and fluoroscopy (Figure 1C). Once transseptal puncture is performed and confirmed on TEE, the RF pigtail wire is advanced into the LA and used as a rail for the steerable sheath and dilator (Figure 1D). The steerable sheath is deflected towards the mitral annulus (Figure 2A) and the pigtail wire is, then, advanced into the apex of the LV (Figure 2B). In patients with mitral stenosis, thickening of the mitral leaflets may encumber access for the pigtail wire; in this case, a 6F multipurpose guide catheter can be introduced through the steerable sheath to facilitate LV access.
Figure 1: ransseptal puncture workflow for ViV-TMVR procedures using VersaCross steerable system. Fluoroscopy image of A) VersaCross steerable system used to cannulate the SVC, and B) Placement of VersaCross steerable system septum for precise transseptal puncture C) TEE image of tenting of septum (yellow arrow; LA=Left Atrium, RA=Right Atrium, SVC=Superior Vena Cava) D) Fluoroscopy image of RF pigtail wire and steerable sheath across the septum into the LA.
Mitral valve deployment
The RF pigtail wire is used to maintain LV access, while the steerable sheath is exchanged for a 14 mm × 40 mm Atlas Percutaneous Transluminal Angioplasty Balloon (Gold, Bard, Temple, USA) is used to dilate the interatrial septum as well as the mitral valve (Figure 2C). Several passes with a slightly inflated balloon is used to flosses the intra-atrial septum, as well as the mitral valve annulus to insure easy passage of the delivery system. After withdrawing the balloon, the Edwards SAPIEN 3 Transcatheter Heart Valve System (Edwards LifeSciences Corp) is advanced into the Inferior Vena Cava (IVC), with the valve mounted on the Commander delivery catheter with the skirt towards the handle. The SAPIEN 3 valve is then loaded on the Edwards balloon catheter, and the sheath is rotated 180 degrees clockwise. The SAPIEN 3 valve and balloon assembly are advanced across the mitral valve with the VersaCross RF wire serving as a rail (Figure 2D). The balloon is slowly inflated for subsequent deployment (Figure 2E), followed by balloon deflation, and removal the Commander delivery device and VersaCross RF wire.
Figure 2: Fluoroscopy images of ViV-TMVR workflow using VersaCross steerable system and SAPIEN 3 Transcatheter Heart Valve System. Using VersaCross steerable sheath, the RF pigtail wire is A) Steered towards the mitral valve and B) Positioned into the LV. Use of RF pigtail wire as exchange rail for subsequent steps including C) Interatrial septal balloon dilation D) SAPIEN 3 delivery system advancing over the RF pigtail wire, E) Valve deployment-balloon fully expanded.
Discussion
Significant progress has been made in transcatheter treatment of valvular heart disease in the past decade such as the establishment of Transcatheter Aortic Valve Replacement (TAVR) as a therapeutic option for patients with severe aortic stenosis in all surgical risk profiles [9,10]. Although ViV-TMVR is at an earlier stage in its development as a therapeutic option, it offers a reliable and robust alternative to patients who suffer from bioprosthetic mitral valve degeneration and are deemed at high risk for repeat mitral valve surgery [2]. Advancement in transseptal catheterization has allowed for ViV-TMVR procedures becoming an increasingly favorable option for both physicians and patients [6].
Although ViV-TMVR is the least invasive approach, it is technically challenging requiring precise TSP for effective device delivery. Most ViV-TMVR patients involve patients who have undergone previous transseptal therapies or who are considered of older age which may present with resistant septa [11]. Additionally, TSP site specificity plays an important role in the success of the procedural outcomes, whereby non-optimal TSP location can result in downstream procedural difficulties and complications. The RF needle have previously been shown to improve TSP without employing significant mechanical force by using RF energy, improving TSP efficiency, and reducing complications [12,13]. The VersaCross steerable system, comprised of a RF transseptal wire and steerable sheath, similarly uses RF energy to facilitate TSP with minimal mechanical force. The operator can adjust the puncture site for more precise TSP by tracking back to the SVC without any guidewire exchanges. The RF wire eliminates the need for guidewire or needle exchanges, which may reduce the risk of air embolism or tissue injury [14,15]. Additionally, TSP can be done directly with the steerable sheath for more precise and controlled TSP, required to navigate the tight boundaries of the LA in order to reach the mitral valve.
Approximately 50% of patients experience mitral valve stenosis, which may encumber cannulation of the LV with a guidewire; requiring an additional exchange of catheter and guidewire, to advance through the mitral valve [6]. Typically following a successful TSP and LA catheterization, the transseptal needle and fixed curve sheath and dilator are exchanged for a steerable sheath, such as an Agilis steerable sheath (Abbott, St. Paul, Mn, USA), to guide a stiff exchange guidewire into the LV to provide a support rail for the valve delivery sheath, and device deployment. Our current practice has streamlined the device delivery workflow by utilizing a single wire-based steerable system for both transseptal puncture and mitral valve catheterization. The atraumatic pigtail RF wire provides a compliant bumper during dropdown from the SVC and positioning on the septum to enable safe TSP with the VersaCross steerable sheath. Without necessitating extra guidewire exchanges, the same pigtail RF wire allows for the pigtail to be anchored in the LV without losing access throughout the duration of the procedure or getting caught up in the mitral valve chordae. Additionally, the steerable sheath can be adequately controlled to allow precise navigation of the mitral annulus and enable support of the pigtail wire.
In our experience, transseptal access was successful using the technique described in this paper with no peri-procedural complications. We have also experienced improved procedural efficiency, whereby transseptal access is typically obtained within 3 minutes using the VersaCross RF wire and significantly improved relative to alternative transseptal methods (5 min) during transcatheter mitral valve repair [14]. This is consistent with previously reported transseptal times using the VersaCross RF wire system and significantly improved relative to alternative transseptal methods (16 minutes) during transcatheter mitral valve repair [14,16,17].
Conclusion
Although, surgical mitral valve replacement is currently recognized as the standard of care for patients with mitral valve disease when transcatheter mitral valve repair is not indicated, the procedure is associated with a higher rate of surgical risk and procedural complications. In our experience, efforts to streamline workflows can improve safety and access to ViV-TMVR procedures in patients with degenerative mitral valve bioprostheses at high-risk for repeat surgical mitral valve replacement. This includes the adoption of wire-based transseptal systems (e.g. VersaCross steerable system) to reduce the number of steps and device exchanges. A comprehensive analysis is warranted to establish robust long-term data.
Acknowledgements
We acknowledge Rhodaba Ebady and Saja Al-Dujaili (Boston Scientific, Scientific Affairs) for their assistance in manuscript preparation.
Funding
This work did not receive external funding.
Competing and Conflict of Interests
The authors have no competing and conflict of interests to declare.
References
- Testa L, Rubbio AP, Casenghi M, et al. Transcatheter mitral valve replacement in the transcatheter aortic valve replacement era . J Am Heart Assoc. 8(22): e013352 (2019).
- Harloff MT, Chowdhury M, Hirji SA, et al. A step-by-step guide to transseptal valve-in-valve transcatheter mitral valve replacement. Ann Cardiothorac Surg. 10(1): 113-121 (2021).
- Head SJ, Celik M, Kappetein AP, et al. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 38(28): 2183-2191 (2017).
- Ejiofor JI, Hirji SA, Ramirez-Del F, et al. Outcomes of repeat mitral valve replacement in patients with prior mitral surgery: A benchmark for transcatheter approaches. J Thorac Cardiovasc Surg. 156(2): 619-627 (2018).
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 121(16): 1848-1857 (2010).
- Whisenant B, Kapadia SR, Eleid MF, et al. One-year outcomes of mitral valve-in-valve using the SAPIEN 3 transcatheter heart valve. JAMA Cardiol. 5(11): 1245-1252 (2020).
- Alkhouli M, Rihal CS, Holmes DR, et al. Transseptal techniques for emerging structural heart interventions. JACC Cardiovasc Interv. 9(24): 2465-2480 (2016).
- Alperi A, Garcia S, Rodes-Cabau J, et al. Transcatheter valve-in-valve implantation in degenerated surgical aortic and mitral bioprosthesis: Current state and future perspectives. Prog Cardiovasc Dis. 72: 54-65 (2022)
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 380(18): 1695-1705 (2019).
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 380(18): 1706-1715 (2019).
- Urena M, Himbert D, Brochet E, et al. Transseptal transcatheter mitral valve replacement using balloon-expandable transcatheter heart valves: A step-by-step approach. JACC Cardiovasc Interv. 10(19): 1905-1919 (2017).
- Smelley MP, Shah DP, Weisberg I, et al. Initial experience using a radiofrequency powered transseptal needle. J Cardiovasc Electrophysiol. 21(4): 423-427 (2010).
- Winkle RA, Mead RH, Engel G, et al. The use of a radiofrequency needle improves the safety and efficacy of transseptal puncture for atrial fibrillation ablation. Heart Rhythm. 8(9):1411-1415 (2011).
- Sayah N, Simon F, Garceau P, et al. Initial clinical experience with VersaCross transseptal system for transcatheter mitral valve repair. Catheter Cardiovasc Interv. 97(6): 1230-1234 (2021).
- Anselmino M, Matta M, Toso E, et al. Silent cerebral embolism during atrial fibrillation ablation: Pathophysiology, prevention and management. J Atr Fibrillation. 6(2): 796 (2013).
- Inohara T, Gilhofer T, Luong C, et al. VersaCross radiofrequency system reduces time to left atrial access versus conventional mechanical needle. J Interv Card Electrophysiol. 63(1): 9-12 (2022).
- Maisano F, La Canna G, Latib A, et al. Transseptal access for MitraClip® procedures using surgical diathermy under echocardiographic guidance. EuroIntervention. 8(5): 579-586 (2012).