Special Report - Imaging in Medicine (2012) Volume 4, Issue 1
Using functional MRI to study auditory comprehension
Javier Gonzalez-Castillo1, Olumide A Olulade2 & Thomas M Talavage*31Section on Functional Imaging Methods, Laboratory of Brain & Cognition, National Institute of Mental Health, NIH, Bethesda, MD 20892, USA
2Center for the Study of Learning, Georgetown University Medical Center, Washington, DC 20057, USA
3School of Electrical & Computer Engineering, Weldon School of Biomedical Engineering, Purdue University, 465 Northwestern Ave, West Lafayette, IN 47907, USA
- Corresponding Author:
- Thomas M Talavage
School of Electrical & Computer Engineering
Weldon School of Biomedical Engineering
Purdue University, 465 Northwestern Ave
West Lafayette, IN 47907, USA
Tel: +1 765 494 5475
Fax: +1 765 494 3358
E-mail: tmt@purdue.edu
Abstract
Over the past 20 years, functional MRI (fMRI) has proven to be a powerful tool for the investigation of cognitive behavior in the brain. One of the more challenging aspects of the use of this technique is the application to studies involving acoustic stimulation, given that the measurement process generates acoustic noise of high intensity that can mask or otherwise impede perception and comprehension of a desired auditory stimulus. Advances in attenuation, acquisition strategies and experimental design have enhanced the use of fMRI in this context, with recent advances in active noise cancellation and our understanding of the fMRI-assessed hemodynamic response laying a framework for continued improvement.
Keywords
acoustic noise ▪ auditory cortex ▪ experimental design ▪ functional MRI ▪ language ▪ speech
Speech, in contrast with other high-order cognitive functions, such as attention or emotion, is unique to humans. For many years the lack of a proper animal model restricted the study of the neuronal correlates of speech perception to lesion studies such as those performed by Broca [1] or Wernicke [2], or intra- and postoperative electrocortical recording sessions, such as those conducted by Penfield et al. [3], Ojemann [4] and Howard et al. [5]. It is the development of modern neuroimaging techniques, such as EEG, magnetoencephalography, PET and most recently functional MRI (fMRI), that has allowed neuroscientists to study how the brain processes speech in vivo with minimal – in the case of PET – or no risk for the subject.
Of all these, fMRI, being a completely noninvasive neuroimaging technique with excellent combined spatial and temporal resolution, is particularly well suited for the study of highly distributed functions such as speech. Successful application of fMRI to the study of auditory comprehension has permitted not only the understanding of how the temporal cortex contributes to different stages of auditory comprehension [6–8], but it has significantly added to the development of the dual-stream model of speech perception, which establishes that understanding of spoken language is the result of collaborative processing in two parallel streams: a ventral stream that is involved in mapping sound onto meaning, and a dorsal stream that is involved in mapping sound onto articulatory-based representations [9–11].
In spite of its successful application to auditory comprehension, the fMRI scanning environment imposes three major restrictions on the experimental tasks and paradigms that may be used. First, MRI scanners are highly confined spaces, which limits the ways in which the experimenter can interact with the subjects. Second, signal changes of interest (e.g., those indicative of neuronal activity) are on the same order of magnitude as the measurement noise, ultimately extending the time period required for data collection to yield robust results. Finally, and of extreme importance for the study of auditory function, MRI scanners are noisy equipment that make delivery of auditory stimuli far from a simple endeavor. It is the consequences of this last restriction that we focus on in this article.
We first describe the different sources of acoustic noise present in the environment. In the ‘Confounds associated with imaging-related acoustic noise’ section we discuss the potential confounding effects that elevated noise levels may have on the interpretation of fMRI results. In the ‘Attenuation of imaging-related acoustic noise’ section, we introduce the reader to some of the most commonly used techniques to overcome the difficulty of delivering auditory stimuli in the presence of loud continuous noise in the confinement of the scanner bore. We end with some conclusions and future directions on how fMRI will help us advance our understanding of the neuronal mechanisms of speech perception.
Acoustic noise sources in fMRI
There are two main sources of acoustic noise in the scanner room: the cold head compressor in charge of recondensing helium so that the temperature of the superconducting coils is kept below 4.2 K (the boiling point of helium); and the sound derived from the fast switching of gradient fields characteristic of most fMRI acquisition sequences. Other minor noise sources (e.g., the patient cooling fan and hum from fluorescent lighting) will be site-specific, but are generally not of experimental significance.
The cold head compressor produces a continuous low-frequency, relatively low-intensity pumping sound at all times, independent of whether scanning is in progress. This noise source primarily interferes with communication with subjects via the patient intercom. Still, it is worth noting that the low-level noise from the compressor has reported to affect patterns of activation within the inferior colliculus [12]. If necessary the compressor can be switched off for short periods of time, negating its confounding effects.
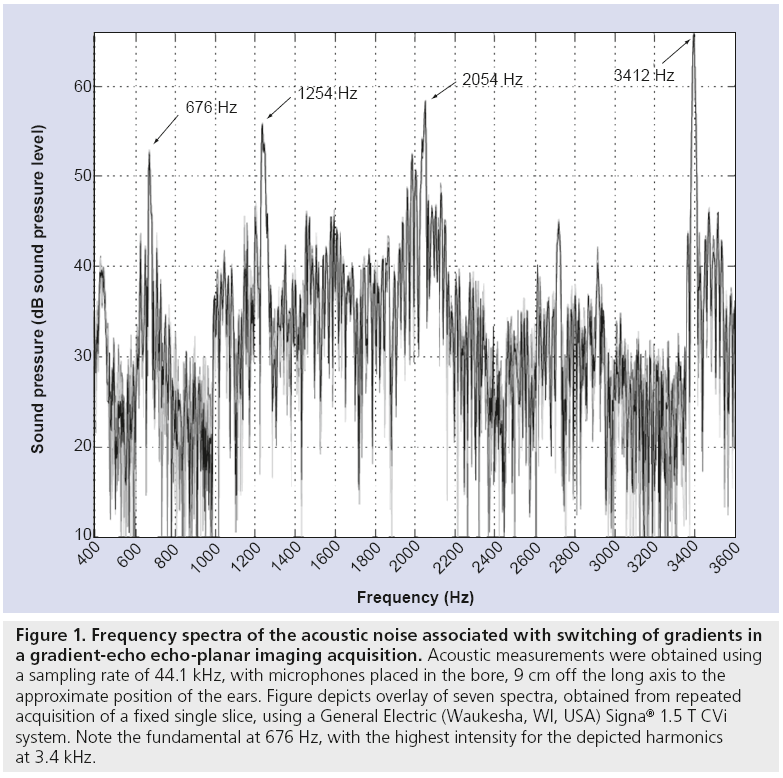
By contrast, the sound generated by switching of the gradient fields during acquisition time is a high-intensity sound having a broad frequency spectrum – fundamental frequency typically between 0.5 and 2 kHz and significant harmonics above 10 kHz (Figure 1). This second sound, which is the one of the main concerns for the fMRI experimenter, is especially intense in the bore of the magnet where the head of the subject sits, generally peaking between 94 and 135 dB sound pressure level, depending on imaging system characteristics and the chosen imaging sequence [13,14].
Figure 1: Frequency spectra of the acoustic noise associated with switching of gradients in a gradient-echo echo-planar imaging acquisition. Acoustic measurements were obtained using a sampling rate of 44.1 kHz, with microphones placed in the bore, 9 cm off the long axis to the approximate position of the ears. Figure depicts overlay of seven spectra, obtained from repeated acquisition of a fixed single slice, using a General Electric (Waukesha, WI, USA) Signa® 1.5 T CVi system. Note the fundamental at 676 Hz, with the highest intensity for the depicted harmonics at 3.4 kHz.
Confounds associated with imaging-related acoustic noise
Acoustic noise in fMRI experimentation represents an undesired source of auditory stimulation for the subject [15,16], and therefore, a potential confound in the data [17] (see [18,19] for additional reviews). Loud noises, such as the one produced by echo-planar imaging acquisitions, may shift the subject’s attention from the experimental task and decrease sensory perception or comprehension of the auditory stimulus of interest (e.g., speech, music, tones). In addition, the loud noise can reduce subject comfort, limiting the duration of experiments and quality of results.
As an example, attention is known to significantly affect patterns of activation observed with fMRI. Higher attention demands are usually correlated with larger and more reliable activations [20], as well as the recruitment of additional cortical regions [21]. In the particular case of auditory comprehension, presence of elevated acoustic noise levels – forcing subjects to increase attention to segregate the target from noise – has been associated with recruitment of additional areas in the medial occipital cortex and adjacent cerebellar cortex [22–24].
In addition to sensory and cognitive confounds, exposure to continuous acoustic noise causes a baseline elevation of the fMRI-detectable signal in auditory areas. As a result of this elevated baseline, the dynamic range for activation due to auditory stimuli of interest is reduced when compared with a silent environment [25]. This reduced range translates to decreases in measured signal change, associated statistical values and the number of significantly active auditory cortex voxels arising from experimental stimulation [26,27]. Greater reductions occur for progressively longer durations [28,29] or higher duty cycles of scanner noise [30].
As the interaction between acoustic imaging noise and fMRI activations is not linear [25,31,32], it is extremely difficult to attempt removal of acoustic noise effects during data analysis. As a result, most of the effort in combating acoustic noise during imaging has focused on the reduction of noise levels at the subject’s ear. Some of these methods are described below.
Attenuation of imaging-related acoustic noise
Improvements in scanner manufacturing, such as gradient copper shielding [33,34], have helped reduce sound levels inherent in structural scanning, producing, as a by-product, a less hostile environment for fMRI. However, while attenuation to minimum noise levels of 50–65 dB sound pressure level has been achieved by these methods, higher sound levels remain present for the techniques used in fMRI. Other changes to the hardware with the potential to reduce acoustic imaging noise include using heavier gradient coils that are less susceptible to Lorentz forces, placing gradient coils in a vacuum to avoid noise transmission through the surrounding air, as well as the use of rubber dampeners and foam insulation around the gradient coils [13,35]. These approaches provide attenuation of 10–30 dB, and while they are relatively expensive solutions they represent the majority of approaches adopted by MRI manufacturers.
Another approach to reducing noise levels includes passive and active attenuation applied at the level of the patient. Traditional attenuation techniques have involved the use of earplugs and circumaural ear muffs to attenuate the sound intensity at the subjects’ ears. These can reduce the noise by up to 35 dB, but while this results in a safer environment for the subject, the level of attenuation provided by this method is generally insufficient to prevent acoustic masking of the presented stimulus and may hamper communication with the subject during the experiment. Moreover, while the use of earplugs is effective at reducing high-frequency components of the noise, lowfrequency components are not well attenuated owing to bone conduction [36].
Perhaps a more sophisticated and versatile approach to acoustic noise reduction at the subject’s ear is the use of active noise cancellation. The underlying principle here is the simultaneous presentation of antiphase noise that competes destructively with noise components of the MRI scanner [37–39]. This method is effective at reducing low-frequency components of the noise [40]; however, attenuating high-frequency components remains a challenge. Nevertheless, a recent study has shown that active noise cancellation during auditory comprehension experiments can be successful at reducing parietofrontal activation believed to represent additional effort necessary for discrimination of auditory stimuli in a noisy environment [41].
In addition to enhanced attenuation, researchers have sought to develop ‘silent’ pulse sequences via manipulation of the gradients [42–44]. These have provided as much as 40 dB of attenuation, but are relatively ineffective for fast-imaging sequences such as the commonly used echo-planar imaging sequence. To account for this, researchers have combined these silent pulse sequences with parallel imaging techniques [45–47] such as sensitivity encoding (SENSE [48]), representing a promising approach to obtaining a quiet MRI environment while maintaining good temporal resolution. Rather than developing pulse sequences that provide silent periods, a different approach involves the use of continuous-sound gradient pulse sequences that exploit the cortical preference for transient sounds [49]. Such sequences have been demonstrated to result in a lower baseline and an increased blood oxygen level-dependent amplitude when compared with conventional fMRI sequences [50].
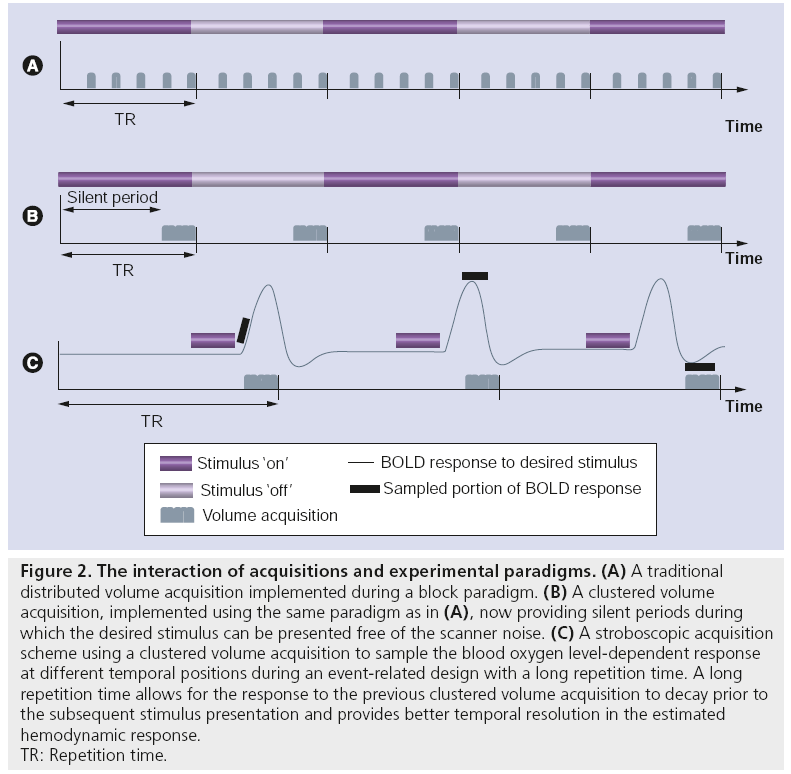
Perhaps the most common experimental method to combat acoustic noise has been the use of acquisition protocols that provide short periods of silence between successive acquisitions. Such protocols include clustered volume acquisition techniques [27] for use in sparse scanning paradigms [51], providing (potentially long) intervals of quiet during which a stimulus can be presented free from scanner noise (Figure 2). In the ideal case, data acquisition is set to temporally coincide with the peak of the hemodynamic response to the desired stimulus [52]. Clustered volume acquisitions may also be utilized with stroboscopic acquisition techniques in event-related paradigms [53,54]. Such techniques involve time-shifting (i.e., jittering) the stimulus presentation within the silent window period to allow sampling of the blood oxygen level-dependent response at various temporal locations using a fixed repetition time. This method provides better temporal resolution of the obtained hemodynamic response via post hoc trial sorting. While these methods have significant benefits in reducing the effect of acoustic imaging noise (especially for detection of activation in the auditory cortex [41,55]), residual overlap of responses to ambient noise and auditory stimuli still represents an important confound. One way to avoid this is by the use of extremely long sampling periods (up to 20 s between acquisitions [54]). This extended period potentially allows the response to the acoustic noise to decay prior to stimulus presentation, such that the response to the latter is measured in isolation. Such experimental designs, however, result in an inefficient trade-off between net duration and obtained statistical power.
Figure 2: The interaction of acquisitions and experimental paradigms. (A) A traditional distributed volume acquisition implemented during a block paradigm. (B) A clustered volume acquisition, implemented using the same paradigm as in (A), now providing silent periods during which the desired stimulus can be presented free of the scanner noise. (C) A stroboscopic acquisition scheme using a clustered volume acquisition to sample the blood oxygen level-dependent response at different temporal positions during an event-related design with a long repetition time. A long repetition time allows for the response to the previous clustered volume acquisition to decay prior to the subsequent stimulus presentation and provides better temporal resolution in the estimated hemodynamic response. TR: Repetition time.
Conclusion
Despite the MRI scanning environment not being optimal for the performance of auditory tasks owing to its elevated acoustic noise levels, fMRI has significantly contributed over the years to advancing our understanding of the neuronal correlates of auditory comprehension. Improvements leading to quieter hardware, more efficient attenuation techniques, and acquisition sequences optimized for delivery of auditory stimuli during short periods of silence have allowed neuroscientists to use fMRI to understand the different processing stages that are required to successfully decode spoken utterances.
Future perspective
Today, in great part due to research conducted with fMRI, we know that auditory comprehension involves a set of bilaterally distributed regions that extend well beyond those initially considered in the classical models proposed by Wernicke [2] or Geschwind [56]. Moreover, by means of carefully designed fMRI experiments, we have a rough understanding of the different roles that many temporal, parietal and frontal areas play in translating auditory inputs to the primary auditory cortex into meaningful concepts (for a review, see [57]). Still, our understanding of auditory comprehension is not complete as elementary questions, such as how simple acoustic features of pitch or intensity are processed and how languagerelevant signals (e.g., linguistic tones [58]) are selectively routed to the hemisphere dominant for language, are still open to debate. Future applications of fMRI in the realm of auditory comprehension will not only include finding conclusive answers to these questions, but also in helping clinicians determine language laterality accurately with significantly lower risks [59], or the creation of detailed individualized maps of eloquent cortex prior to surgical interventions [60] that will help boost the prognosis of such interventions.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Broca PP. [Perte de la parole, ramolissement chronique et destruction partielle du lobe antérieur gauche du cerveau]. Bull. de la Societe Anthropologique 2, 235–238 (1861). Wernicke C. [Der Aphasische Symptomenkomplex: Eine Psychologische Studie auf Anatomischer Basis] (The Aphasia Symptom-complex: A Psychological Study on an Anatomical Basis). M. Cohn and Weigert, Breslau, Poland (1874).
- Penfield W, Roberts L. Speech and Brain Mechanisms. Princeton University Press, Princeton, NJ, USA (1959).
- Ojemann GA. Brain organization for language from the perspective of electrical-stimulation mapping. Behav. Brain Sci. 6(2), 189–206 (1983).
- Howard MA, Volkov IO, Mirsky R et al. Auditory cortex on the human posterior superior temporal gyrus. J. Comp. Neurol. 416(1), 79–92 (2000).
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J. Neurosci. 17(1), 353–362 (1997).
- Binder JR, Frost JA, Hammeke TA et al. Human temporal lobe activation by speech and nonspeech sounds. Cereb. Cortex 10(5), 512–528 (2000).
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123(Pt 12), 2400–2406 (2000).
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition 92(1–2), 67–99 (2004).
- Scott SK, Johnsrude IS. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 26(2), 100–107 (2003).
- Scott SK, Wise RJ. The functional neuroanatomy of prelexical processing in speech perception. Cognition 92(1–2), 13–45 (2004).
- Melcher JR, Levine RA, Bergevin C, Norris B. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear. Res. 257(1–2), 63–74 (2009).
- Foster JR, Hall DA, Summerfield AQ, Palmer AR, Bowtell RW. Sound-level measurements and calculations of safe noise dosage during EPI at 3 T. J. Magnet. Reson. Imaging 12(1), 157–163 (2000).
- Ravicz ME, Melcher JR, Kiang NY. Acoustic noise during functional magnetic resonance imaging. J. Acoustical Soc. Am. 108(4), 1683–1696 (2000).
- Bandettini PA, Jesmanowicz A, Van Kylen J, Birn RM, Hyde JS. Functional MRI of brain activation induced by scanner acoustic noise. Magn. Reson. Med. 39(3), 410–416 (1998).
- Tamer GG Jr, Luh WM, Talavage TM. Characterizing response to elemental unit of acoustic imaging noise: an fMRI study. IEEE Trans. Biomed. Eng. 56(7), 1919–1928 (2009).
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage 27(2), 377–386 (2005).
- Amaro E Jr, Williams SC, Shergill SS et al. Acoustic noise and functional magnetic resonance imaging: current strategies and future prospects. J. Magn. Reson. Imaging 16(5), 497–510 (2002).
- Moelker A, Pattynama PM. Acoustic noise concerns in functional magnetic resonance imaging. Hum. Brain Mapp. 20(3), 123–141 (2003).
- Specht K, Willmes K, Shah NJ, Jancke L. Assessment of reliability in functional imaging studies. J. Magn. Reson. Imaging 17(4), 463–471 (2003).
- Hugdahl K, Thomsen T, Ersland L, Rimol LM, Niemi J. The effects of attention on speech perception: an fMRI study. Brain Lang. 85(1), 37–48 (2003).
- Gonzalez-Castillo J, Talavage TM. Reproducibility of fMRI activations associated with auditory sentence comprehension. Neuroimage 54(3), 2138–2155 (2011).
- Binder JR, Liebenthal E, Possing ET, Medler DA, Ward BD. Neural correlates of sensory and decision processes in auditory object identification. Nat. Neurosci. 7(3), 295–301 (2004).
- Salvi RJ, Lockwood AH, Frisina RD, Coad ML, Wack DS, Frisina DR. PET imaging of the normal human auditory system: responses to speech in quiet and in background noise. Hear. Res. 170(1–2), 96–106 (2002).
- Talavage TM, Edmister WB. Nonlinearity of fMRI responses in human auditory cortex. Hum. Brain Mapp. 22(3), 216–228 (2004).
- Scarff CJ, Dort JC, Eggermont JJ, Goodyear BG. The effect of MR scanner noise on auditory cortex activity using fMRI. Hum. Brain Mapp. 22(4), 341–349 (2004).
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM. Improved auditory cortex imaging using clustered volume acquisitions. Hum. Brain Mapp. 7(2), 89–97 (1999).
- Shah NJ, Jancke L, Grosse-Ruyken ML, Muller-Gartner HW. Influence of acoustic masking noise in fMRI of the auditory cortex during phonetic discrimination. J. Magn. Reson. Imaging 9(1), 19–25 (1999).
- Hu S, Olulade O, Castillo JG et al. Modeling hemodynamic responses in auditory cortex at 1.5 T using variable duration imaging acoustic noise. Neuroimage 49(4), 3027–3038 (2010).
- Olulade O, Hu S, Gonzalez-Castillo J et al. Assessment of temporal state-dependent interactions between auditory fMRI responses to desired and undesired acoustic sources. Hear. Res. 277(1–2), 67–77 (2011).
- Gaab N, Gabrieli JD, Glover GH. Assessing the influence of scanner background noise on auditory processing. I. An fMRI study comparing three experimental designs with varying degrees of scanner noise. Hum. Brain Mapp. 28(8), 703–720 (2007).
- Langers DR, Van Dijk P, Backes WH. Interactions between hemodynamic responses to scanner acoustic noise and auditory stimuli in functional magnetic resonance imaging. Magn. Reson. Med. 53(1), 49–60 (2005).
- Edelstein WA, Hedeen RA, Mallozzi RP, El-Hamamsy SA, Ackermann RA, Havens TJ. Making MRI quieter. Magn. Reson. Imaging 20(2), 155–163 (2002).
- Edelstein WA, Kidane TK, Taracila V et al. Active-passive gradient shielding for MRI acoustic noise reduction. Magn. Reson. Med. 53(5), 1013–1017 (2005).
- Katsunuma A, Takamori H, Sakakura Y, Hamamura Y, Ogo Y, Katayama R. Quiet MRI with novel acoustic noise reduction. Magma 13(3), 139–144 (2002).
- Ravicz ME, Melcher JR. Isolating the auditory system from acoustic noise during functional magnetic resonance imaging: examination of noise conduction through the ear canal, head, and body. J. Acoust. Soc. Am. 109(1), 216–231 (2001).
- Goldman AM, Gossman WE, Friedlander PC. Reduction of sound levels with antinoise in MR imaging. Radiology 173(2), 549–550 (1989).
- Chen CK, Chiueh TD, Chen JH. Active cancellation system of acoustic noise in MR imaging. IEEE Trans. Biomed. Eng. 46(2), 186–191 (1999).
- McJury M, Stewart RW, Crawford D, Toma E. The use of active noise control (ANC) to reduce acoustic noise generated during MRI scanning: some initial results. Magn. Reson. Imaging 15(3), 319–322 (1997).
- Hall DA, Chambers J, Akeroyd MA, Foster JR, Coxon R, Palmer AR. Acoustic, psychophysical, and neuroimaging measurements of the effectiveness of active cancellation during auditory functional magnetic resonance imaging. J. Acoust. Soc. Am. 125(1), 347–359 (2009).
- Blackman GA, Hall DA. Reducing the effects of background noise during auditory functional magnetic resonance imaging of speech processing: qualitative and quantitative comparisons between two image acquisition schemes and noise cancellation. J. Speech Lang. Hear. Res. 54(2), 693–704 (2011).
- Hennel F, Girard F, Loenneker T. ‘Silent’ MRI with soft gradient pulses. Magn. Reson. Med. 42(1), 6–10 (1999).
- Girard F, Marcar VL, Hennel F, Martin E. Anatomic MR images obtained with silent sequences. Radiology 216(3), 900–902 (2000).
- Chapman BLW, Haywood B, Mansfield P. Optimized gradient pulse for use with EPI employing active acoustic control. Magn. Reson. Med. 50(5), 931–935 (2003).
- Hennel F. Fast spin echo and fast gradient echo MRI with low acoustic noise. J. Magn. Reson. Imaging 13(6), 960–966 (2001).
- de Zwart JA, van Gelderen P, Kellman P, Duyn JH. Reduction of gradient acoustic noise in MRI using SENSE‑EPI. Neuroimage 16(4), 1151–1155 (2002).
- Loenneker T, Hennel F, Ludwig U, Hennig J. Silent BOLD imaging. Magn. Reson. Mater. Phy. 13(2), 76–81 (2001).
- Boesiger P, Pruessmann KP, Weiger M, Scheidegger MB. SENSE: sensitivity encoding for fast MRI. Magn. Reson. Med. 42(5), 952–962 (1999).
- Harms MP, Guinan JJ Jr, Sigalovsky IS, Melcher JR. Short-term sound temporal envelope characteristics determine multisecond time patterns of activity in human auditory cortex as shown by fMRI. J. Neurophysiol. 93(1), 210–222 (2005).
- Seifritz E, Di Salle F, Esposito F, Herdener M, Neuhoff JG, Scheffler K. Enhancing BOLD response in the auditory system by neurophysiologically tuned fMRI sequence. Neuroimage 29(3), 1013–1022 (2006).
- Hall DA, Haggard MP, Akeroyd MA et al. ‘Sparse’ temporal sampling in auditory fMRI. Hum. Brain Mapp. 7(3), 213–223 (1999).
- Eden GF, Joseph JE, Brown HE, Brown CP, Zeffiro TA. Utilizing hemodynamic delay and dispersion to detect fMRI signal change without auditory interference: the behavior interleaved gradients technique. Magn. Reson. Med. 41(1), 13–20 (1999).
- Backes WH, van Dijk P. Simultaneous sampling of event-related BOLD responses in auditory cortex and brainstem. Magn. Reson. Med. 47(1), 90–96 (2002).
- Belin P, Zatorre RJ, Hoge R, Evans AC, Pike B. Event-related fMRI of the auditory cortex. Neuroimage 10(4), 417–429 (1999).
- Petkov CI, Kayser C, Augath M, Logothetis NK. Optimizing the imaging of the monkey auditory cortex: sparse vs. continuous fMRI. Magn. Reson. Imaging 27(8), 1065–1073 (2009).
- Geschwind N. The organization of language and the brain. Science 170(961), 940–944 (1970).
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann. NY Acad. Sci. 1191, 62–88 (2010).
- Xu Y, Gandour J, Talavage T et al. Activation of the left planum temporale in pitch processing is shaped by language experience. Hum. Brain Mapp. 27(2), 173–183 (2006).
- Carlson C. Wada you do for language: fMRI and language lateralization? Epilepsy Curr. 10(4), 86–88 (2010).
- Krings T, Schreckenberger M, Rohde V et al. Functional MRI and 18F FDG-positron emission tomography for presurgical planning: comparison with electrical cortical stimulation. Acta Neurochir. 144(9), 889–899 (2002).
• Key overview of the functional organization of language comprehension.
• Key overview of the functional organization of language comprehension.
• Valuable illustration of how sensitive functional MRI (fMRI) of the auditory pathway is to acoustic noise.
• • Seminal work on enhancing application of fMRI for auditory neuroscience.
• Current state-of-the-art findings regarding active noise cancellation in fMRI.
• An innovative approach to minimizing the effects of imaging-related acoustic noise on auditory fMRI.
• • Seminal work in enhancing the application of fMRI for auditory neuroscience.