Research Article - Interventional Cardiology (2024) Volume 16, Issue 1
Utility of bionanocellulose 1 in reconstruction of the thoracic aorta: An in vivo animal study
- Corresponding Author:
- Piotr Siondalski
Department of Cardiosurgery, Medical University of GdaÅsk, GdaÅsk, Poland,
E-mail: piotr.siondalski@gumed.edu.pl
Received date: 13-Feb-2024, Manuscript No. FMIC-24-127535; Editor assigned: 15-Feb-2024, PreQC No. FMIC-24-127535 (PQ); Reviewed date: 29-Feb-2024, QC No. FMIC-24-127535; Revised date: 07-Apr-2024, Manuscript No. FMIC-24-127535 (R); Published date: 14-Apr-2024, DOI: 10.37532/1755-5310.2024.16(1).775.
Abstract
The aim of this research was to demonstrate the utility of Bacterial Nanocellulose 1 (BNC1) as a new biological material for aortic reconstruction. This paper presents the results of the implantation of aortic prostheses sewn from BNC1 in pigs as a largeanimal model. A fragment of the descending aorta was excised and reconstructed from BNC1 in 16 pigs. Postoperative observations were carried out for 6 months, including assessment of the animals’ general condition and a list of inflammation blood tests. None of the six inflammatory blood parameters was elevated. After 6 months, all the animals were sacrificed. The reconstructed aorta along with the BNC1 prosthesis were excised and underwent macroscopic, histological, and physicochemical evaluation. BNC1 was found to possess features that qualify it as a biological aortic prosthetic material: Excellent surgical suitability and biocompatibility.
Keywords
Bacterial nanocellulose; Aortic tube; Vessel grafts; Cardiac surgery; Reconstruction of the aorta; Porcine model
Introduction
The search for an ideal bioimplant for aortic surgery is currently underway in a vast number of studies conducted across the world [1-3]. One of the most potential materials is Bacterial Cellulose (BC), which is both inexpensive and easily available and may offer optimal parameters according to encouraging initial reports from multiple studies [4,5]. Our earlier multicenter study, conducted between 2013 and 2017, developed a modified BC named Bacterial Nanocellulose 1 (BNC1), with lower thrombogenicity and improved resistance to biodegradation and physical damage [6]. Specifically, BNC1 (produced by Bowil-Biotech, Władysławowo, Poland) is characterized by greater durability than animal tissue and good biocompatibility and apyrogenicity. Additionally, owing to its low thrombogenicity and low hemolysis index, it may be applicable in arterial and heart valve prostheses. These optimistic results allowed us to initiate further investigations employing in vivo animal models. The aim of the study was to evaluate the utility of BNC1 prostheses in the reconstruction of an excised part of the thoracic aorta. The similarity between pig and human circulatory systems [7], may allow for conclusions regarding whether this material may be potentially useful in reconstructive vascular surgery in humans.
Aim of the study
The aim of the study was a multimodal evaluation of the usefulness of bionanocellulose BNC1 as a biological prosthesis in reconstructive surgery of the aorta.
Materials and Methods
BNC1 preparation
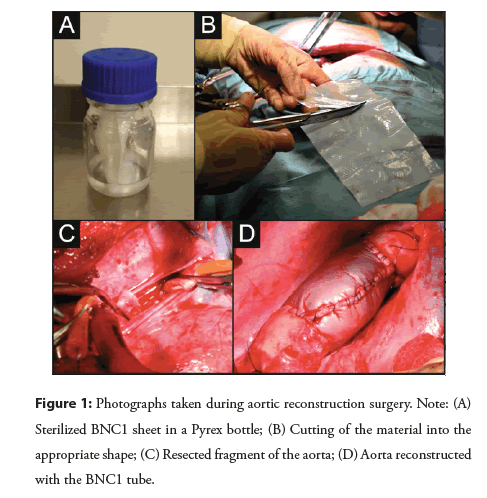
To prepare BNC1, 500 ml of sterile modified Schramm-Hestrin culture medium was instilled under sterile conditions with 50 ml of bacterial suspension prepared two days earlier. The medium was thoroughly mixed and poured onto sterile trays for the cultivation of a stationary film for 5 days in an incubator with gravity air flow at 30°C ± 2°C. Subsequently, the medium surface was covered with a homogeneous film, which was subjected to a multistage purification process. First, using a press, the residual culture medium was removed from the cellulose material, which was then intensively washed with water. It was then treated with a 1% sodium hydroxide solution for a minimum of 1 hour at 80°C; thereafter, 1% acetic acid and purified water were used to neutralize the pH (to the range of 5.5- 7.0). Next, the material was subjected to a thickness adjustment using a press for the target thickness of 2 mm. Then, the cellulose material was cut into 10 × 20 cm pieces, dried at 40°C for 12 hours, and soaked in pyrogen-free water for 2 hours at 20°C ± 5°C. Ultimately, rectangular cellulose films 200 × 100 mm in size and 0.3-0.5 mm in thickness were placed in a Pyrex bottle with a small amount of pyrogen-free water and sterilized at 121°C for 20 minutes in an autoclave (Figure 1A). The ready material was assessed for sterility, level of bacterial endotoxins, and cellulose fiber content.
Animal model
A standardized animal operation scheme was prepared for F1 line DanBred swine, a hybrid of the Danish Great White and Yorkshire breeds. Overall, 16 animals were operated on and observed for 6 months, after which the BNC1 tube was evaluated. The study protocol was approved by the Local Ethics Commission for Research on Animals in Gdańsk, Poland (40/2013; 10/07/2013). The study was performed in accordance with ISO 5840, the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines, and the EU Directive 2010/63/EU regarding animal experiments as shown in the Figure 1.
Operation protocol: All operations were carried out under sterile conditions in accordance with human surgery standards. The animals were operated on under general anesthesia, using drugs for premedication and anesthesia as previously published [8]. The pig was placed on its right side. The skin in the area of the lateral thoracic wall was shaved from the neck to the costal arch and thoroughly disinfected with an alcoholic chlorhexidine solution. The area around the surgical incision was covered with a sterile cloth. The left pleural cavity was opened through the fifth intercostal space. The upper lobe of the left lung was moved toward the diaphragm. An incision was made in the parietal pleura above the ascending aorta, revealing the second artery departing from the aortic arch; the distal segment of the aorta was 10-14 cm long. The outer diameter of the aorta was measured, and two purse strings were placed on the aortic wall at the level of the second artery and approximately 10 cm below. Heparin 5000 U was administered, and Activated Clotting Time (ACT) measurements were performed. At over 300 seconds, the aortic wall was punctured inside the suture, and two 19F arterial cannulas were inserted and connected with a 3/8-inch silicone tube. The two aortic clamps were placed 7 cm apart with the proximal clamp located approximately 2 cm proximally from the proximal cannula. At this point, the blood supply to the lower half of the animal’s body was provided by an aortic-aortic shunt. Then, a 4 cm fragment of the descending aorta was excised between the clamps (Figure 1B). Next, a rectangular BNC1 sheet was cut to match the length and circumference of the excised fragment with 1 cm margins (Figure 1C). The material was connected to the vessel using Prolene 5.0 sutures and a continuous “over-and-over” vascular suturing technique with a distance of approximately 2-3 mm between the punctures. Afterwards, the BNC1 fragment was closed with continuous sutures, creating a tubular prosthesis of the thoracic aorta with a matching diameter (Figure 1D). After removing the distal clamp, the BNC1 prosthesis was de-aired from eventual air bubbles through the unsealed suture line or its puncture; then, the proximal clamp and aortic-aortic shunt were removed. Hemostasis was monitored after the blood supply to the lower body of the pig was restored through the reconstructed aorta. The prosthesis and suture lines were tamped for a few minutes with gauze strips. If necessary, heparinization was reversed with protamine sulfate at a 1:1 ratio. After proper hemostasis was achieved, the parietal pleura was sutured over the prosthesis and the thoracic aorta. The pleural cavity was rinsed with saline solution. When the lungs were expanded, the pleural sheaths were sutured in layers. The air from the pleural cavity was aspirated; after the sutures were sealed, the pleural drain was removed. A sterile dressing was placed over the skin and fastened with single sutures to the animal’s skin. The operative duration ranged between 4 and 6 hours.
BNC1 tube implantation: To determine its potential utility as a vascular prosthesis, the following properties of BNC1 were evaluated during the operation: The ease of cutting and creating appropriate shapes; the ability to form the tubular aortic prosthesis; the extent of stretching during sewing; the susceptibility to damage by surgical threads at the puncture site; the duration of bleeding on the suture line; the eventual occurrence of bleeding, rupture, or unsealing at sites not damaged by sutures after normal blood pressure was restored; the tensile strength after assembly; the blood permeability and ease of piercing with a needle; the transparency of the prosthesis; and the ease of identifying gas bubbles.
Postoperative treatment: Postoperative analgesia was started at skin closure. At the end of the day of the operation, the operated animals were transported back to the farm and placed in separate pens with standard water and forage supply. Postoperative care was delivered by trained technicians and the veterinarian. During the first postoperative week and the recovery period, the animals were assessed in terms of behavior, water and food intake, respiratory rate, and pain symptoms. Additional doses of analgesics were applied if the animals presented symptoms of excessive pain (i.e., groaning, limited movements, anorexia, wavering chest movements on breathing). Postoperative wound site infection prophylaxis was maintained for seven days by the application of penicillin G with streptomycin (Pen-Strep 200+250 mg, Scan-Vet, Gniezno, Poland) at a dose of 0.1 mL/kg given intramuscularly (IM) once a day and by wound disinfection with an alcoholic iodine solution. Additionally, the animals received injections of Calfoset at 0.1 mL/kg IM (Krka, Nove Mesto, Slovenia) for three days to achieve calcium and phosphorus homeostasis.
Assessment of inflammatory reaction after BNC1 bioimplantation during 6 months of observation: The observation time ranged from 172 to 190 days. To monitor the general condition and potential inflammatory response, blood samples were collected according to the following schedule: Before the operation, first month postoperatively, and the 6th month after the operation. We collected blood samples from the right jugular vein in order to carry out laboratory tests: CBC with differential (white blood cells), haematocrit, fibrinogen, CRP and some proand anti-inflammatory cytokines (TNF-α, IL-6 and IL-10).
A control sample was taken from each animal before preoperative drug administration. The following parameters were measured in each sample: White Blood Cell count (WBC); Platelet Count (PLT); Hematocrit (HCT); C-Reactive Protein (CRP); fibrinogen; and cytokines Interleukin 6 (IL-6), interleukin 10 (IL-10) and Tumor Necrosis Factor alpha (TNF-α). Control blood samples were collected from the pigs in their habitat prior to surgery.
Ex vivo rating of BNC1 aortic implant
After 6 months of follow-up after prosthesis implantation, the animals were sacrificed using an anesthetic overdose of 1 mg/kg pentobarbital administered intravenously (Biowet-Puławy, Puławy, Poland). On autopsy, the tissue blocks containing the BNC1 implants were collected and submitted for pathomorphological examination.
Macroscopic evaluation: After the animal was sacrificed, the chest wall was excised together with the scar from the previously performed surgery. The obtained tissue block consisted of skin with layers of integument, pleura and lung fragments, parietal pleura and extra pulmonary space together with the descending aorta reconstructed with BNC1. The obtained tissue block was cut with a dissection knife, and the following parameters were evaluated: Inflammatory infiltrate surrounding the reconstructed aorta, presence of pleural adhesions, endothelial coverage (color, thickness, and gloss of the endothelium covering the BNC1 lumen in comparison to the native aortic lumen), signs of degeneration of the BNC1 prosthesis, and differences in the diameter of the aorta proximal and distal to the implant.
Microscopic evaluation: The sections were collected from the prosthesis-aorta border and fixed in 10% buffered formalin for 48 hours. Then, the sections were dehydrated with the appropriate sequence of alcohol concentrations and xylene (using a Leica processor) and embedded in low-melting-point paraffin. The obtained tissue blocks were cut into 3-5 μm sections and subjected to routine Hematoxylin and Eosin (H&E) staining; 2 to 3 sections per case were examined with an Olympus BX41 microscope equipped with a 10X eyepiece and 2X, 4X, 10X, 20X, 40X, and 60X objectives.
The following features were examined: The degree of inflammatory infiltration (semiquantitative; reflecting the area occupied by inflammatory infiltrate: I°/mild for<25% of the area, II°/moderate for 25%-50% of the area, and III°/severe for>50% of the area), the activity of the inflammatory process, the presence of giant cells, the presence of bone and osteochondral metaplasia (using a two-point scale depending on the presence or absence of given feature), and the thickness of the fibrous tissue covering the outer surface.
In vivo biodegradability of the implants: Five randomly selected specimens were removed from formalin (non-implanted BNC1 samples fixed in formalin were used as controls), washed by shaking at 70 rpm for 2 hours in 100 mL of distilled water with a water change every 15 minutes, and finally freeze-dried. All samples were conditioned prior to analysis for seven days in P4O10.
X-Ray Diffractometry (XRD): XRD was performed using Cu Kα radiation (0.154 nm) and an X’Pert diffractometer (Phillips, Eindhoven, The Netherlands). The diffractometer was operated at 30 mA and 40 kV. The spectra over the range of 4.0-40.0°2θ were recorded at a scan rate of 0.02°2θ/s. The Crystallinity Index (Cr.I.) was calculated based on the XRD measurements according to Segal et al. [9].
Results
Intraoperative evaluation
We faced no difficulties with cutting BNC1 samples into the designed shape with scissors; standard tissue markers were applicable to draw shapes on the material. A tubular shape for an aortic prosthesis was obtained by continuous “over-and-over” suturing of the opposite sides of the rectangle; the material was easily pierceable with a surgical needle. After blood flow restoration through the reconstructed aorta, the blood pressure did not stretch or rupture the material. No bleeding through the material was observed, and the bleeding from the puncture site lasted from 2 to 5 minutes and in most cases resolved before heparin reversal with protamine sulfate. In some cases, pressure was applied with dry gauze pads for several minutes to achieve hemostasis, especially when the sutures were tightened; no local hemostatic preparations or additional sutures were used in any operation. Due to the thin layer of the material and its partial transparency, the effectiveness of de-airing could be monitored as the air bubbles in blood were visible, which was particularly helpful.
The tested material showed excellent material properties, including strength and plasticity, a very good seal could be achieved, and the material could be sewn easily.
The 6 months of observation
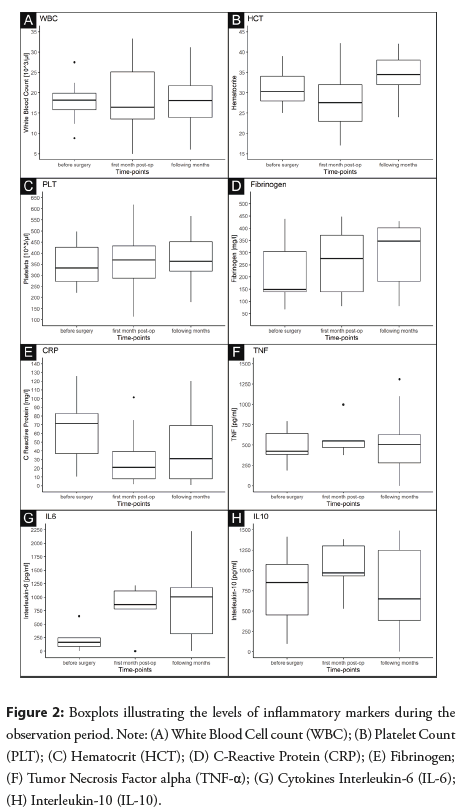
During the observation period. The body temperatures of all animals were within the physiological range-from 39.0 to 39.5°C. No signs of disease or alarming behavior were noted in any animal as shown in the Figure 2.
We noted only minor changes in the complete blood count parameters during the follow-up period (Figures 2A-2C). In healthy pigs, the total number of leukocytes ranged between 10 and 22 × 103/μL. The WBC results were mostly within the normal limits (i.e., 10-22 × 103/µl), with single outlying values (Figure 2A). A slight decrease in hematocrit values was noted in the early postoperative period due to blood loss, which subsequently returned to normal levels (Figure 2B). In turn, the platelet counts were relatively consistent in the lower part of the normal range (i.e., 320-520 × 103/µl) (Figure 2C).
Fibrinogen and CRP: Both proteins were within the normal limits (fibrinogen: 100-500 mg/dl; CRP: 3.6-183 mg/dl) in all measurements (Figures 2D and 2E). A minor elevation of fibrinogen levels was noted during both the early and late postoperative periods (Figure 2D), while a small CRP decrease was found (Figure 2E).
Cytokines: There are no reference values for the investigated cytokines in pigs. We noted some elevation in the IL-6 levels during both the early and late postoperative periods.The other two investigated cytokines showed only minor fluctuations (Figures 2G-2H).
Conclusion: None of the animals showed any alarming behavior during the observation period. All had body temperatures in the physiological range, and none exhibited signs of disease.
Any out-of-range fluctuations in blood parameters were transient and returned to initial values during the early stages of healing. None of these fluctuations indicated any long-term inflammation processes in the operated animals.
BC produced by Gluconacetobacter xylinus E25 showed no inflammatory potential when implanted into the porcine cardiovascular system.
Autopsy
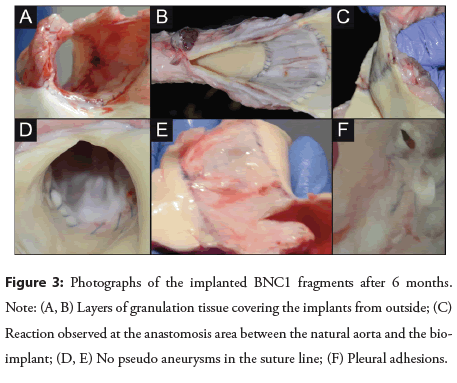
Macroscopic evaluation: There were no macroscopic differences among individual animals in the healing of the implanted prostheses as shown in the Figure 3. We noted a layer of granulation tissue (approximately 0.5-1 cm in thickness) covering the implants from the outside (Figures 3A and 3B). A similar reaction was observed at the anastomosis area between the natural aorta and the bioimplant (Figure 3C). There were no pseudoaneurysms in the suture line (Figures 3D and 3E) or any alterations in the implant diameter (either distension or stenosis). The internal lining of the prostheses was smooth and shiny, resembling the endothelium in the surrounding natural aorta. Some pleural adhesions could be noted (Figure 3F).
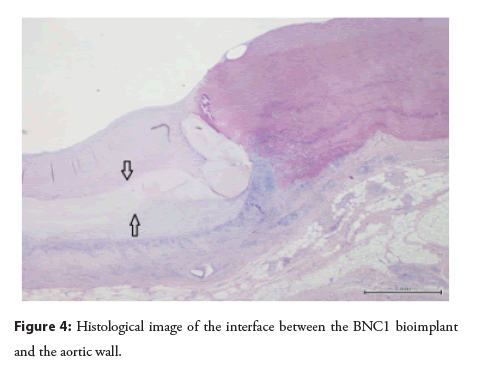
Microscopic evaluation of excised samples: Overall, 37 representative tissue samples were evaluated (between 2 or 3 samples per animal). Chronic inflammatory infiltrates with foreign-body granulomatous reactions were observed in all samples; in 4 samples, the intensity was mild, moderate in 9, and severe in 24. Additionally, in 15 samples, there were foci of metaplastic tissue within the areas of anastomosis with the natural aorta (chondroid metaplasia in 7, osseus in 5, and mixed chondoosseus in 2). Importantly, no immune infiltration into the grafted material could be seen (Figure 4). The luminal side of the implant was covered by pannus (between 0.2 and 2.5 mm in thickness) and lined by endothelium.
Conclusion: Chronic inflammation and granulomatous inflammation with giant cells of the foreign-body type were observed.
There was no penetration of inflammatory cells into the grafted material. Foci of metaplasia were present where BNC1 was anastomosed with the aorta. No other sites of metaplasia were found. The BNC1 prosthesis was covered by fibrous tissue.
Biodegradation of BNC1 implants: Crystallographic and morphological analyses were conducted on the BNC1 implant samples. There were no distinct differences in the shape of XRD diffractograms or in the SEM (Scanning Electron Microscope) images of the tested samples; therefore, only representative diffractograms and images are shown.
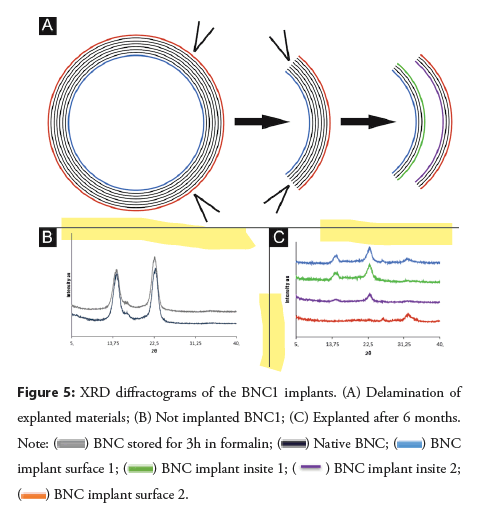
BNC1 implant characterization by XRD: Prior to the analysis, the explanted materials were delaminated to access the internal parts of the material (Figure 5A). As a reference, the native crystalline structure of BNC1 was characterized by two sharp intense peaks at 14.6° and 22.7°2θ (which may be attributed to the Iα and Iβ crystalline forms of cellulose, respectively [10]), as well as a low intensity peak at 16.7° 2θ (Figure 5B). Following formalin treatment, the intensity of the 22.7° peak relatively increased (in comparison to the 14.6° peak), indicating the slight prevalence of the more ordered and more stable Iβ form in the biopolymer [10]. The crystallinity index was not affected by formalin treatment (Table 1). The diffractogram of the BNC1 implant after implantation presented diffraction peaks characteristic of BC on the luminal surface of the implant (surface 1) and its internal parts (inside 1 and inside) (Figure 5C). The shape of the diffractogram of the adventitial surface (surface 2) indicated an amorphic structure (Figure 5B), most likely the surrounding granulation tissue as shown in the Figure 5.
The crystallinity index of the luminal and adventitial surfaces was significantly decreased after the 6-month implantation (Table 1), which likely reflects the growth of pannus and granulation tissue, respectively, in contact with the biomaterial; the Cr.I. of the internal parts was decreased to a lesser extent (Table 1).
| No bleeding (N=52) | No bleeding (N=52) |
|---|---|
| BNC1 implant | Crystallinity index [%] |
| Not implanted | |
| Native | 89 |
| After 3 h in formalin | 88 |
| Explanted after 6 months | |
| Surface 1 | 23 |
| Inside 1 | 63 |
| Inside 2 | 75 |
| Surface 2 | 0 |
Table 1: Crystallinity index of BNC1 implants.
Conclusion: XRD diffractograms and SEM images indicate no biodegradation-related changes in the BNC implants 6 months after implantation in the porcine model. Moreover, based on the research findings, it can be assumed that the material integrates with the surrounding biological tissue, probably the vascular endothelium, which supports its biocompatibility.
Discussion
In this study, we investigated the applicability of a new biomaterial based on BC for the reconstruction of the descending aorta in an in vivo porcine model. BNC1 was selected based on the results of our earlier in vitro studies [6,11-13], indicating its favorable characteristics.
Intraoperatively, the material was easy to suture to the aortic wall with standard sutures used in vascular surgery. We noted some susceptibility to damage by surgical sutures at the puncture site while pulling a single thread. Therefore, adaptative tightening of the prosthesis (optimally with the parachute technique) should be applied. After complete anastomosis, the bioimplant easily took the expected shape and did not stretch after blood flow restoration (despite the high pressure, similar to that in humans), which confirms its adequate plasticity and strength. Complete sealing of the aortic wall was demonstrated with no leakage through the material. The surgical needle punctures made during the suturing sealed spontaneously within a few minutes after releasing the clamps on the aorta. This may be related to the retention of plasma and proteins in the cellulose mesh, which was postulated to provide resistance to infection [14].
On clinical examinations during the 6-month follow-up, we noted good wound healing with no bleeding or aortic flow disturbances. There were no general signs of inflammation (such as fever, pain, and lack of appetite), while the results of laboratory blood tests were within the normal range. These data suggest that the biomaterial was well tolerated by the animals, despite being in constant contact with the blood flowing though the aorta. During the early stages of wound healing, we noted some transient fluctuations in blood parameters, which rapidly returned to baseline levels with no long-term changes. Finally, no decreases in HCT levels during the convalescent period confirm the lack of hemolytic potential of BNC1.
Macroscopic examination of the collected samples showed adequate healing at the interface with the aortic wall as well as internal coverage with the endothelium on the connective tissue stroma, with no aneurysmal dilatation or stenosis. On microscopic examination, the adventitial side of the bioimplant was surrounded by granulation tissue with marked, but variable, chronic inflammatory infiltrate and foreign-body giant cells. This type of reaction occurs in virtually all types of implants [15,16], while its intensity depends on the physical and chemical properties of the biomaterial. The clinical significance of the foreign-body reaction depends on the extent and rate of material degradation [16]. Importantly, in contrast to Gore-Tex and animal biological prostheses [15,17], there was no infiltration of inflammatory cells into BNC1. This is of particular importance, since all degenerative processes are facilitated by immune infiltration into the biomaterial. Additionally, foci of cellular metaplasia were noted in a subset of cases at the interface with the aortic wall. Since metaplasia was present only at sites with dense sutures, we suspect that it may be a reaction to the suture material rather than BNC1. The luminal side of the bioimplant was covered by pannus and lined by endothelium. The thickness of the pannus was related to the distance from the interface with the aortic wall and did not affect blood flow.
Both the XRD diffractograms and the calculated crystallinity index for BNC1 were similar to those reported for other BC materials [18-20].
The XRD diffractograms of samples collected 6 months after implantation showed only non-characteristic changes in the signal, interpreted as secondary to granulation tissue growth at the adventitial surface. The absence of the signal typical for calcification was interpreted as the lack of biodegradation. Moreover, based on the results, it can be concluded that the material integrates with the surrounding biological tissue, which further supports its biocompatibility.
Limitations
This study had a follow-up of 6 months, which limits the inferences on eventual long-term degradation. Unfortunately, the authors were unable to ultimately explain whether the foreignbody reaction at the suture line was due to the sutures themselves or BNC1.
Conclusion
Multifactorial evaluation of the experimentally implanted BNC1 within the descending aorta of pigs proved the excellent suitability of the material used for reconstruction of the aortic wall. The results of this preclinical study of BNC1-the bacterial nanocellulose produced at the certified biopharmacological Bowil Biotech factory-for reconstruction of the aorta support further planning of clinical trials.
Conflict of Interests
The authors declare no conflicts of interest.
Funding
The study was financed by the National Center for Research and Development in Poland (grant No. KARDIO BNC PBS2/ A7/16/2013).
References
- Zhang F, Bambharoliya T, Xie Y, et al. A hybrid vascular graft harnessing the superior mechanical properties of synthetic fibers and the biological performance of collagen filaments. Mater Sci Eng C Mater Biol Appl. 118: 111418 (2020).
- Wang D, Xu Y, Li Q, et al. Artificial small-diameter blood vessels: Materials, fabrication, surface modification, mechanical properties, and bioactive functionalities. J Mater Chem B. 8(9): 1801-122 (2020).
- Nemoto S, Konishi H, Shimada R, et al. In situ tissue regeneration using a warp-knitted fabric in the canine aorta and inferior vena cava. Eur J Cardiothorac Surg. 54(2): 318-327 (2018).
- Bäckdahl H, Risberg B, Gatenholm P, et al. Observations on bacterial cellulose tube formation for application as vascular graft. Mater Sci Eng C. 31(1): 14-21 (2011).
- Czaja W, Krystynowicz A, Bielecki S, et al. Microbial cellulose-the natural power to heal wounds. Biomaterials. 27(2): 145-151 (2006).
- Kołaczkowska M, Siondalski P, Kowalik MM, et al. Assessment of the usefulness of bacterial cellulose produced by Gluconacetobacter xylinus E25 as a new biological implant. Mater Sci Eng C. 97: 302-312 (2019).
- Crick SJ, Sheppard MN, Ho SY, et al. Anatomy of the pig heart: Comparisons with normal human cardiac structure. J Anat. 193(1): 105-119 (1998).
- Kowalik MM, Siondalski P, Kolaczkowska M, et al. Challenges in using anesthesia for open chest and aorta surgery in swine. Med Weter. 76(09): 517-524 (2020).
- Segal L, Creely J, Martin Jr A, et al. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J. 29(10): 786-794 (1959).
- Saska S, Teixeira LN, de Oliveira PT, et al. Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J Mater Chem. 22(41): 22102-22112 (2012).
- Gawlikowski M, Janiczak K, Zawidlak-WB, et al. Selected in vitro study of bionanocellulose toward its utilization in medical devices contacting with blood. Engineering of Biomaterials. (2016).
- Dawidowska K, Stanis A. Influence of preservative on the tensile strength of the tissue of porcine circulatory system. Adv Mater Sci. 15(3): 67-75 (2015).
- Stanis A, Staroszczyk H, Szkodo M, et al. The effect of dehydration/rehydration of bacterial nanocellulose on its tensile strength and physicochemical properties. Carbohydr Polym. 236: 116023 (2020).
- Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals. 7(5): 545-594 (2014).
- Anderson JM, Rodriguez A, Chang DT, et al. Foreign body reaction to biomaterials. Semin Immunol. 20(12): 86-100 (2008).
- Sheikh Z, Brooks PJ, Barzilay O, et al. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials. 8(9): 5671-701 (2015).
- Glagov S. Intimal hyperplasia, vascular modelling, and the restenosis problem. Circulation. 89(6): 2888-2891 (1994).
- Shi X, Cui Q, Zheng Y, et al. Effect of selective oxidation of bacterial cellulose on degradability in phosphate buffer solution and their affinity for epidermal cell attachment. RSC Advances. 4(105): 60749-60756 (2014).
- Zhijiang C, Guang Y. Bacterial cellulose/collagen composite: Characterization and first evaluation of cytocompatibility. J Appl Polym Sci. 120(5): 2938-2944 (2011).
- Hu Y, Zhu Y, Zhou X, et al. Bio-absorbable cellulose composites prepared by an improved mineral-binding process for bone defect repair. J Mater Chem B. 4(7): 1235-1246 (2016).