Perspective - Interventional Cardiology (2012) Volume 4, Issue 5
Varicose veins: evaluating modern treatments, with emphasis on powered phlebectomy for branch varicosities
- Corresponding Author:
- Thomas W Wakefield
Section of Vascular Surgery, University of Michigan Medical School, CVC 5463
1500 E. Medical Center Drive SPC 5867, Ann Arbor, MI 48109-5048, USA
Tel: +1 734 936 5820
Fax: +1 734 647 9867
E-mail: thomasww@umich.edu
Abstract
Keywords
ablation, TIPP, varicose veins
Varicose veins: epidemiology, risk factors & pathophysiology
Chronic venous insufficiency (CVI) is one of the most commonly reported medical conditions in the USA with healthcare costs of associated venous ulcers exceeding US$1 billion annually [1]. The underlying pathophysiology of CVI is venous hypertension of the lower extremities, which can lead to various clinical problems including pain, dilated or varicose veins, swelling, edema, skin changes and ulcerations. Although CVI-associated ulcers only affect up to 1% of the population, less severe manifestations of CVI, such as varicose veins, carry a prevalence of 2–56% worldwide [2]. Varicose veins should not be confused with reticular veins or telangiectasias as the treatment modalities differ. Varicose veins are dilated, palpable subcutaneous veins generally larger than 3 mm; reticular veins are dilated nonpalpable subdermal veins 1–3 mm in size; telangiectasias are dilated intradermal venules less than 1 mm [3]. While millions of individuals seek medical attention annually for cosmetic management of their varicose veins, as an early clinical finding on the continuum of CVI, treatment of symptomatic varicose veins is indicated for reasons other than appearance.
Risk factors associated with CVI and varicose veins have been well described. A strong familial relationship for varicose veins has been demonstrated in multiple studies [2,4–8]. However, with the exception of a few congenital disorders associated with varicose veins (e.g., Klippel–Trenaunay syndrome and Chuvash polycythemia), no specific gene has been identified with the development of varicose veins [4]. Increased age and female gender have also been demonstrated to be linked to the development of varicose veins in large epidemiological studies [9,10]. Furthermore, multiparous women have been shown to have a higher risk of developing varicose veins over time, independent of pregnancy-associated weight gain [6,11]. However, obese women are three-times more likely than nonoverweight women to develop and report varicose veins while no such relationship has been shown for men [12]. Finally, occupations that require long periods of standing have been associated with the development of varicose veins [2,13,14].
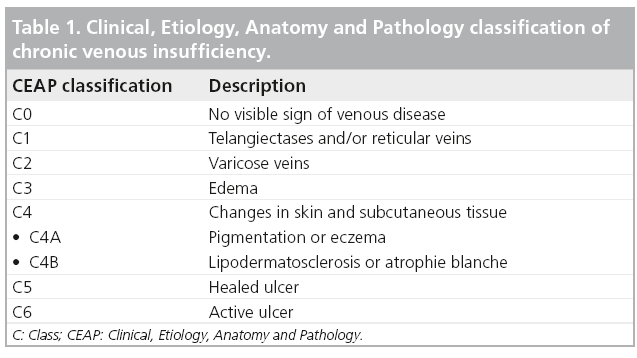
The discomfort of varicose veins is often described as fatigue, heaviness or even itching, all of which can be exacerbated following prolonged standing and relieved by leg elevation. However, associated lower extremity edema and hyperpigmentation of the skin are common. In order to create a common objective language by which the clinical manifestations, distribution and underlying pathophysiology of CVI could be universally understood the Clinical, Etiology, Anatomic and Pathophysiology (CEAP) classification of CVI was developed (Table 1). CEAP was created by an international consensus to provide uniformity in reporting, diagnosing and treating CVI [3].
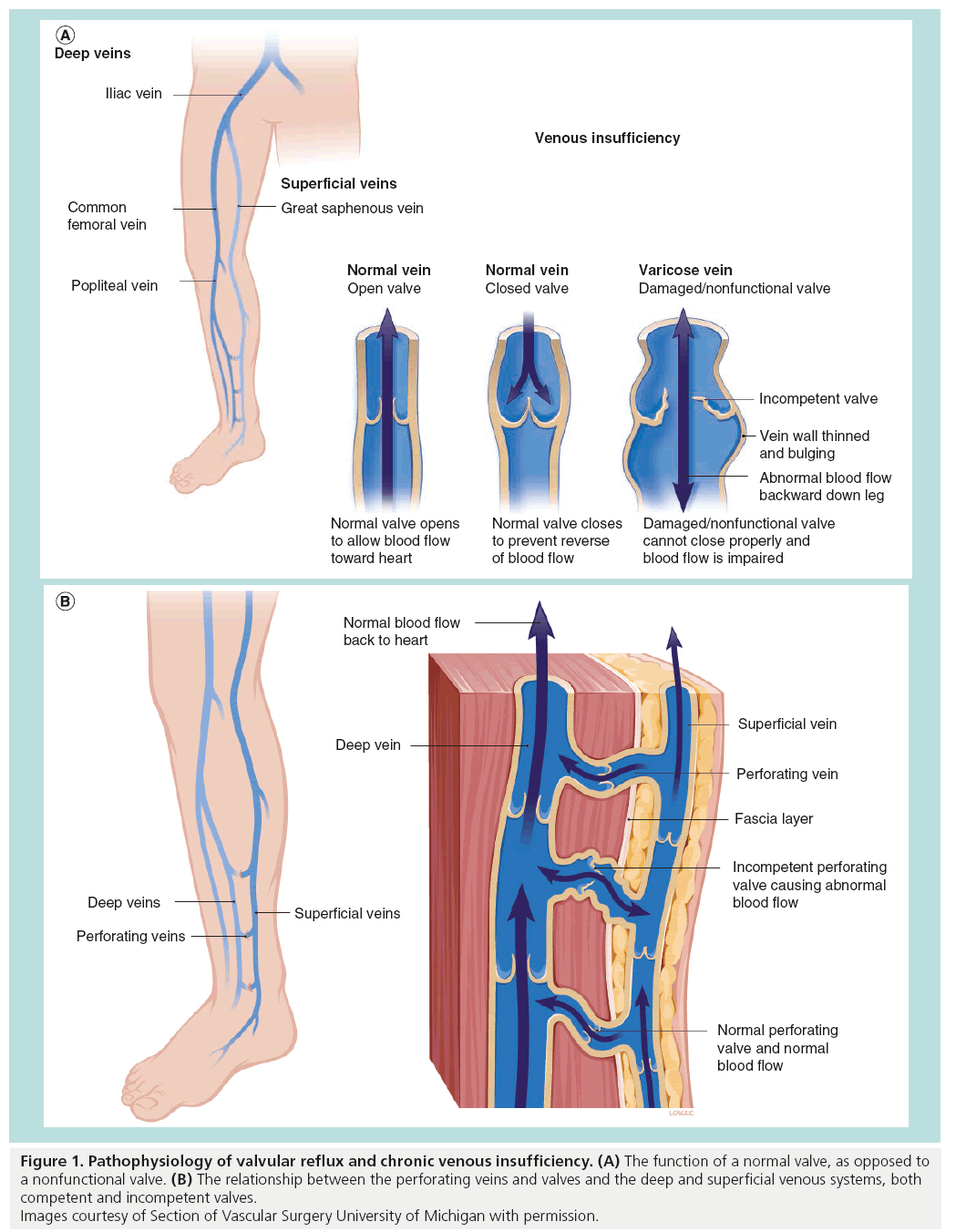
The venous anatomy of the lower leg is made up of two axial systems, a superficial and a deep venous system. The superficial system lies above the muscle fascia in the subcutaneous tissues. The principal veins in the superficial system are the great saphenous vein (GSV) and the small saphenous vein (SSV). The GSV runs from the medial ankle to the groin, joining the common femoral vein at the saphenofemoral junction. The SSV runs from the lateral ankle to the level of the knee where it drains into the popliteal vein at the saphenopopliteal junction. The axial deep veins lie deep within the fascial compartments and run in parallel with the major named arterial structures. The below knee deep vein coalesce to form the popliteal vein, followed by the femoral vein, common femoral vein and iliac vein. In addition, the profunda femoris vein drains the upper leg and serves as a tributary to the common femoral vein. The deep and superficial systems are interconnected by fascial piercing perforator veins. It is generally regarded that the deep system is a system of high pressure as it is subject to the calf muscle pump responsible for pumping blood back to the heart. As pressure increases during calf muscle contraction, blood is forced upwards toward the heart. A series of one-way bicuspid valves prevents refluxing of blood back into the leg. As blood is ejected from the deep veins, venous pressure drops allowing blood to flow from the superficial system through the perforator veins, once again filling the deep system. During calf muscle contraction, one-way valves in the perforator veins prevent back flow of blood into the superficial system (Figure 1A).
Figure 1: Pathophysiology of valvular reflux and chronic venous insufficiency. (A) The function of a normal valve, as opposed to a nonfunctional valve. (B) The relationship between the perforating veins and valves and the deep and superficial venous systems, both competent and incompetent valves. Images courtesy of Section of Vascular Surgery University of Michigan with permission.
CVI occurs when this normal flow of blood back to the heart is disrupted secondary to venous valve incompetence or venous obstruction. Valvular incompetence can occur at all three levels, deep, superficial and perforator. When valvular reflux occurs at the saphenofemoral junction, saphenopopliteal junction or perforator veins, high pressure occurs in the superficial system. High pressure within the superficial system leads to venous dilation, which contributes to superficial valve incompetence. As a result of superficial valve incompetence, high pressure is transmitted to small subcutaneous veins, which become dilated tortuous clusters of varicose veins (Figure 1B).
Although valve ref lux contributes to the underlying pathophysiology of varicose veins, the cause of primary valve failure is less understood. There is no consensus as to whether primary valve incompetence is the inciting event in varicose veins or rather incompetence results from persistent vein wall dilation. Clearly, there are abnormalities in the vein wall in varicose veins that may be independent of valvular reflux and be related to their development. Secondary valve failure may occur in the setting of direct injury, phlebitis or venous hypertension due to proximal obstruction. In this setting, proximal obstruction may be the result of compression from the May–Thurner syndrome, or deep venous thrombosis. Independent of obstruction, deep venous thrombosis has the potential to cause secondary valve failure by direct damage to the valve or fibrosis of the vein wall, preventing the ability of the valve mechanism to remain competent [1].
Treatment options for superficial vein reflux
As described above, the underlying pathologic process behind the formation of varicose veins lies within both deep and superficial reflux. Conventional teaching suggests that the underlying truncal or axial reflux must be treated in order to achieve success with superficial varicosities. Currently, treatment of deep axial reflux is limited to compression therapy with stockings, good exercise (e.g., walking, biking or swimming) and intermittent leg elevation. Although techniques of autologous valve transplant, autologous valve creation and surgical bypass have been described as therapeutic options for deep system reflux, no randomized trial has shown them to be more beneficial that compression. However, in the setting of superficial GSV and SSV or perforator reflux, surgical options exist.
Historically, treatment of superficial system reflux centered on surgical disconnection of the GSV or SSV from its respective insertion into the common femoral or popliteal vein. This was followed by stripping the vein from its surrounding subcutaneous tissues to allow for removal. However, endovenous catheter treatment has made surgical stripping a procedure of the past. Endovenous ablation of the saphenous vein has become so successful that it has been assigned a grade 1B recommendation by the Society of Vascular Surgery [15]. In a large meta-analysis, both endovenous ablation and surgical stripping are statistically equivalent at 3 months with regard to efficacy and recurrence [16–18]. However, the less invasive endovascular ablation technique is less painful and allows for a quicker return to baseline function, making it superior to open surgery [16]. In a similar fashion, treatment of incompetent perforators can also be performed using endovascular catheter-based techniques.
Once a decision has been made to treat the superficial axial reflux, the timing of when to treat the associated varicose veins is controversial. Treatment of the varicosities may be done concomitantly at the time of superficial venous ablation, or alternatively, the procedure may be staged and varicosities can be removed at a later date. The primary advantage of performing a staged procedure is that treating the axial reflux of the superficial system may be sufficient in the select patient to cause regression of the smaller varicosities and resolution of all symptoms. However, in those patients who do not experience resolution, they will incur the need for an additional procedure. By contrast, performing both ablation and varicose vein phlebectomy concomitantly reduces the risk of residual varicosities [19], but may subject some patients to a more extensive procedure than they actually need.
Currently, superficial ablation can be achieved using either a radiofrequency ablation catheter (RFA) or an endovenous laser catheter (EVLT). Randomized controlled trials have attempted to demonstrate a difference and establish superiority between the two therapies. EVLT may be more efficacious at ablating the GSV, although, with the exception of one trial by Gale et al., this has been an insignificant observational trend [20–22]. Additionally, most studies have used the first-generation RFA technology, rather than the new improved second-generation RFA catheter system. There is strong evidence that RFA is superior to EVLT in regards to postoperative pain and bruising [23,24]. However, these trials were done with earlier laser technology using an 810‑nm or 980‑nm wavelength laser. Newer catheters that use a 1470‑nm wavelength laser have a theoretical benefit of causing less bruising [25]. Regardless, all studies listed above show that in long-term follow-up, the immediate postoperative advantages of RFA over EVLT are not continuous, with both procedures offering equal cosmesis by 3 months.
At our institution we often use the secondgeneration ClosureFAST™ radiofrequency ablation catheter (VNUS ClosureFAST; VNUS Medical Technologies, CA, USA) in order to achieve great saphenous vein closure. Prior to sterile prepping the course of the great saphenous vein in visualized in real time with ultrasound and marked with permanent ink. This guides the surgeon in both insertion of the ablation catheter, as well as focal administration of tumescence anesthesia. The great saphenous vein is accessed using a Seldinger technique at the level of, or just below, the knee. A 7-French sheath is placed into the vein over a guide wire. Through this sheath, the catheter is introduced into the great saphenous vein. The catheter is 60 or 100 cm long; however the active ablation tip is 7 cm long. Under ultrasound guidance, the catheter is advanced towards the saphenofemoral junction. The tip of the catheter is highly echogenic allowing for easy visualization. To prevent inappropriate transfer of energy to the common femoral vein, the catheter is pulled back at least 2 cm from the saphenofemoral junction. Under ultrasound guidance, tumescent anesthesia is then rapidly delivered via a pump to the perivenous tissue surrounding the great saphenous vein. Liberal use of tumescence decreases pain, hydrodissects the vein away from surrounding tissues and allows an efficient transfer of energy during vein ablation. After the patient is placed in the Trendelenburg position, the vein is ablated by delivering a 20 s RFA treatment to the vein wall and then pulling the catheter back in stages. The first ablation is repeated twice due to the large diameter of the proximal great saphenous vein. At the completion of vein closure, ultrasonography is used to confirm both ablation as well as patency of the common femoral vein. A similar procedure is performed for the small saphenous vein, taking care to be at least 2 cm from the saphenopopliteal junction.
Modern treatment of branch varicosities
Just as ablative catheter techniques have made open ligation and stripping a historic procedure, new techniques in the removal of varicose veins are challenging traditional stab phlebectomy. In the past 10 years, a more contemporary approach for the treatment of varicosities, known as transilluminated powered phlebectomy (TIPP), has evolved. While TIPP offers a relatively new technique for varicose vein removal, traditional ambulatory phlebectomy is the gold standard for varicose vein removal and does not require the use of special equipment other than a scalpel and vein hook. Varicose veins are marked preoperatively and small incisions are made along the course of the vein. A small hook is placed under the skin and the varicose vein is pulled from the subcutaneous tissues. In a sequential fashion, the vein is removed through a series of small incisions. Commercial transillumination devices exist to assist in locating the vein; however, this is not necessary in most cases. Once the proximal and distal ends of the varicose vein are reached, the vein can be ligated and allowed to retract back into the subcutaneous tissues. These small incisions are rarely closed by sutures but routinely left open or, more often, closed with steri-strips. With adequate local anesthesia, ambulatory phlebectomy can be performed in an office-based setting with or without conscious sedation.
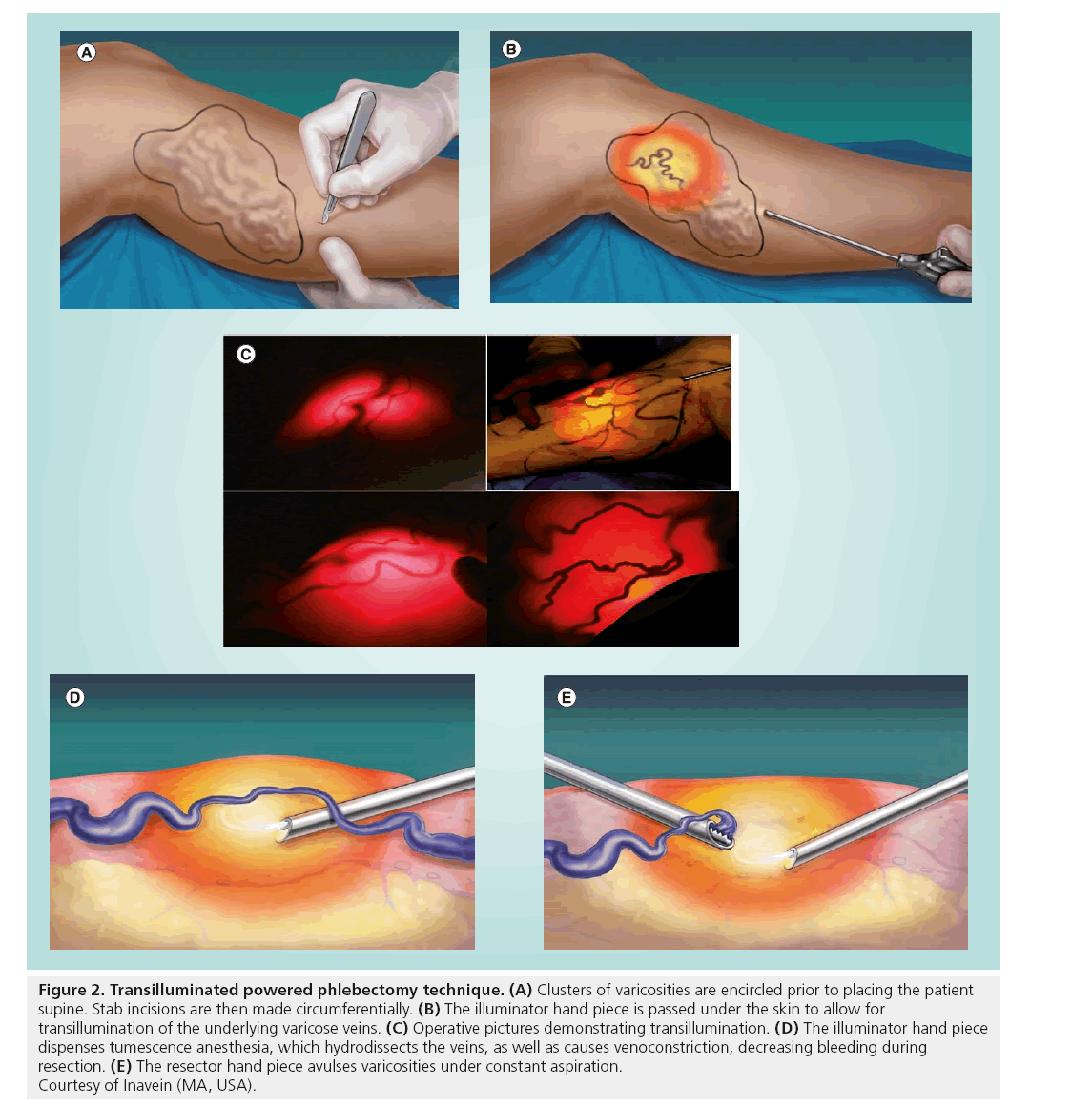
TIPP (Trivex™, Inavein, MA, USA) is an alternative technique for the removal of varicose veins and may be especially useful for removal of larger clusters of varicosities. Theoretical advantages of TIPP include a decrease in the number of incisions, removal of veins under direct visualization and a perceived faster technique for removal of varicose veins, especially large clusters. The Trivex system is made of a central tower with controls for xenon light source, irrigation pump and resection oscillation speeds. The tower provides two handpieces, an illuminator handpiece, which is also capable of hydrodissecting surrounding tissues with tumescence irrigation, and a resectorhandpiece, which is available in a two sizes, 4.5 and 5.5 mm. During a TIPP procedure, the handpieces are introduced through small incisions placed circumferentially around clusters of varicosities (Figure 2A). Proper transillumination is achieved by passing the illuminator handpiece into the subcutaneous tissues beneath varicose veins, allowing for transillumination and visualization of these veins onto the surface of the skin (Figure 2B & C). Placement of the illuminated handpiece into the deep or superficial tissues limits transillumination. Constant administration of tumescence from the illuminator hand piece hydrodissects the vein, which also complements removal (Figure 2D). Resection is done in line with the course of the vein, resecting the varicosities furthest away first and then working towards oneself (Figure 2E). Counter tension is applied to bring the vein taught, aiding in removal. Care is taken during vein resection to avoid passing the resector hand piece too superficially, which can lead to skin tears. Conversely, placing the resector too deeply may increase postoperative paresthesias, as well as limit the ability for proper vein resection. Small 1.5-mm punch incisions are made to allow for drainage of blood and tumescence that collects in the vein tract, limiting postoperative hematoma and bruising [25,26].
Figure 2: Transilluminated powered phlebectomy technique. (A) Clusters of varicosities are encircled prior to placing the patient supine. Stab incisions are then made circumferentially. (B) The illuminator hand piece is passed under the skin to allow for transillumination of the underlying varicose veins. (C) Operative pictures demonstrating transillumination. (D) The illuminator hand piece dispenses tumescence anesthesia, which hydrodissects the veins, as well as causes venoconstriction, decreasing bleeding during resection. (E) The resector hand piece avulses varicosities under constant aspiration. Courtesy of Inavein (MA, USA).
Following resection, the leg is repetitively compressed removing all blood and tumescence. Stab incisions are closed with steri-strips. We advocate for multiple layers of compression along the entire course of the ablated GSV and areas of phlebectomy comprised of heavy absorbent pads, followed by short stretch bandages and finally, an elastic stretch bandage, which is applied from the foot to the groin.
Patients are seen back in the clinic within 6–10 days. Duplex ultrasound is used to confirm ablation of the GSV as well as identify subclinical hematomas or fluid collections. At this visit, bruising is expected as it has not resolved in greater than 60% of people by this time. One must be careful in examining the patient’s bruising at this stage as fading ecchymoses are often mistaken for cellulits. However, true infectious complications are quite rare and reoperation for abscess or infected fluid collection occurs in far less than 1% the population. Patients are transitioned from their compression bandages to a compression stocking of 30–40 mmHg.
Although supporters of TIPP credit shorter operative times and fewer incisions as primary benefits over ambulatory phlebectomy, opponents site a higher incidence of postoperative bruising and hematoma, paresthesias and cost without a difference in cosmetic appearance or clinical outcome. Early criticisms of TIPP centered around a high learning curve in order to avoid skin tears and excessive ecchymosis. Since first entering clinical use in 2000, there have been a limited number of studies, either prospective or retrospective, regarding the outcome of TIPP [27]. Included are three small randomized trials comparing TIPP with ambulatory phlebectomy [28–30]. All three trials documented fewer incisions with TIPP; however, the benefits of a shorter operative time were overshadowed by a steep learning curve [31]. Only one of these studies attempted to compare the impact that either procedure had on the quality of life postoperatively [30]. In this small trial, quality of life assessment was conducted during short-term follow-up at 1 and 6 weeks. Although patients in the TIPP cohort demonstrated a reduction in early quality of life, this trial was small, with only 29 patients included in the TIPP cohort.
Although current data do not establish superiority of TIPP over ambulatory phlebectomy, one must consider that these trials evaluated a new technology in its infancy. Most published literature uses a device and operative technique hindered by higher oscillation frequency (800–1200 rpm) and an approach of minimal tumescence irrigation secondary to not using a punch drainage technique. Newer generation TIPP systems and technical modifications have incorporated a lower oscillation frequency (300–500 rpm), dermal punch drainage technique, smaller and serrated resector head and a recommendation of copious administration of tumescence irrigation. With these adjustments TIPP has become less traumatic, decreasing potential complications and improving outcomes previously reported. Furthermore, it must be highlighted that all early trials treated concomitant superficial venous incompetence with an open saphenofemoral ligation and vein stripping. In performing simultaneous procedures, it is difficult to ascertain the effect of one procedure on the outcome of the other, especially when referring to postoperative pain and quality of life. As catheter-based ablation of the great saphenous vein is now recommended and the modified TIPP technique has not been evaluated in a contemporary trial, previous literature regarding the outcomes of TIPP is outdated. The decision to offer stab phlebectomy or TIPP lies within patient selection. Anecdotally, we feel patients with large clusters of varicosities are better served by TIPP, while those patients with few isolated varicosities may be best served by stab phlebectomy. In choosing either procedure, one must consider the venue in which the procedure will be performed, in an operating room or in an officebased procedure room. This decision is strongly influenced on the type of anesthesia, which will be required to provide adequate sedation and analgesia during the procedure. Traditionally, we have advocated for general anesthesia during a combined GSV ablation and extensive (greater than 20 stab incisions) phlebectomy. However, performing phlebectomy in an ambulatory setting using just local tumescence anesthesia is acceptable. Long procedural time and patient discomfort may limit the extent of phlebectomy that can be done using just tumescence anesthesia in the office setting. Therefore, when an extensive phlebectomy is anticipated in the office setting, multiple phlebectomy procedure sessions may be required to achieve acceptable results. In select patients who have required extensive phlebectomy, but the risk of general anesthesia is high, we have found success in conscious sedation and local tumescence anesthesia.
When performing TIPP for extensive varicosities, we use a similar strategy with regards to anesthesia; the majority of patients undergo general or spinal anesthesia. Unlike stab phlebectomy, consideration must be given to the discomfort of passing the illuminator and resector hand pieces through the subcutaneous tissues. In the select patient, we have found that conscious sedation with copious amounts of tumescence anesthesia is possible. The applicability of office-based TIPP procedures has been recently demonstrated by Spitz who published his singlecenter office-based TIPP experience [32]. This small series highlights 36 patients who underwent a TIPP procedure in the office setting using only tumescence anesthesia. Patients in this series were thin (mean BMI: 25 kg/m2) and young (mean age: 58.8 years), with the majority having only CEAP-2 disease severity (64%). Furthermore, the majority of the cohort only had one cluster of veins to remove. At 3 months there was no reportable hematoma, deep venous thrombosis or extended paresthesias. Although ambulatory TIPP may not be possible for all patients, Spitz has demonstrated that with careful patient selection, the future of TIPP includes the office venue.
To date, the largest published TIPP experience includes 339 patients, of which 88% required just 20 or fewer incisions [33]. At 12 weeks, there were no significant hematomas and no recurrent varicose veins. Patient satisfaction with clinical outcome was stated at 99.7%. Despite good reported subjective outcomes, this large case series did not address postoperative paresthesias of the operative leg, which is often cited as one of the largest disadvantages of TIPP. The incidence of cutaneous nerve injury causing paresthesias with TIPP has been reported as high as 38% [26,27]. However, it is important to consider that stab phlebectomy can also cause postoperative paresthesias, with an incidence as high as 25% in one series [29]. Furthermore, as TIPP is often performed simultaneously with GSV ablation, one must consider the paresthesias that result from the ablative catheter, independent of phlebectomy. Estimated rates of paresthesias following RFA of the GSV range from 2.8 to 33% [34]. In our practice, patients who have undergone an RFA treatment of the GSV in the past 12 months report a 12.3% incidence in paresthesias postoperatively (14 out of 114). This is most likely, in part, secondary to transfer of energy from the ablative catheter to the saphenous and sural nerves. In our experience with TIPP during the same time period, we report a 26% incidence of postoperative paresthesias (34 out of 131). However, as most of these patients also underwent a simultaneous RFA procedure, it is difficult to accurately ascertain the incidence of paresthesias attributable to the TIPP procedure. In addition, in our experience, the incidence is lower using the smaller 4.5-mm head rather than the larger 5.5-mm head (21 vs 31%). Nonetheless, in those patients who maintained regular postoperative follow-up or who were compliant with a telephone interview, resolution of paresthesias occurred in 30% of the cases, usually within 6 months. Furthermore, only one patient thought the paresthesias were bothersome to the point of regretting surgery. Importantly, using the TIPP approach rather than traditional stab phlebectomy, the number of incisions per procedure has decreased in our practice from 31 to eight.
Conclusion
Although stab phlebectomy remains the gold standard for removal of varicose veins, the introduction of TIPP into the surgical armamentarium for varicose veins has allowed for shorter operative times and fewer stab incisions. The greatest benefit of TIPP is seen when an extensive phlebectomy is needed. In experienced hands, the benefits of TIPP may allow for improved clinical outcomes and patient satisfaction. However, there has yet to be a trial comparing the modern TIPP technique to stab phlebectomy.
Initial bruising following the procedure is common and self-limiting. Postoperative bruising seems to decrease with both the use of the 4.5-mm serrated head on the resector hand piece and as the learning curve is conquered. The long-term cosmetic results are well accepted, as stab incisions leave small 2–3 mm scars and punch incisions heal as small freckles on the skin.
Future perspective
The future direction of TIPP is unknown. The paucity of supportive data and lack of widespread use have limited its solidification as a tool in the armamentarium against venous disease. Current evidence does not offer a superior benefit of TIPP over stab phlebectomy. Still, after surmounting the learning curve, the use of TIPP is definitely not harmful and, anecdotally, may serve its best purpose in removing large extensive clusters of varicosities. Advances and modifications in TIPP over the past decade have created a more refined and precise procedure and with the recent introduction of the 4.5 mm serrated head, there is no reason to suspect the technology will not continue to improve. In order for TIPP to thrive, comparative trials are necessary. It is already known that TIPP decreases the number of necessary incisions, but does this matter clinically or economically? Does the decreased procedure time equate into an economic advantage? Do the paresthesias with TIPP occur at a greater frequency or severity that with stab phlebectomy? Furthermore, are such paresthesias even a clinical consequence?
All of these queries need to be examined before judgment can be passed on the future of TIPP. However, in experienced hands, the benefits of TIPP allow the patient to experience a quicker procedure with fewer incisions and given the use of subcutaneous transillumination, a decrease in missed varicosities. Although office-based TIPP is in its infancy, early results look promising. As with all emerging technologies, the key to success is careful patient selection as well as patience in learning and understanding a new technique. If these simple principles are adhered to, incorporating TIPP into the algorithm for treatment of varicose veins, especially large clusters, is a possibility for all venous surgeons.
Executive summary
Varicose veins can be an early manifestation of chronic venous disease
▪ Risk factors include advanced age, female gender, multiparity and occupational predisposition.
▪ Additional symptoms included leg heaviness and fatigue, edema and hyperpigmentation.
▪ The Clinical, Etiology, Anatomy and Pathology classification is a standardized way to compare chronic venous disease amongst studies and across institutions.
Valvular dysfunction & venous reflux can occur within the superficial, deep & perforator systems
▪ High pressure within the superficial system is transmitted to small subcutaneous veins, which can become dilated and tortuous varicosities.
Treatment options for superficial reflux
▪ Surgical ligation and stripping.
▪ Endovenous laser therapy.
▪ Radiofrequency ablation.
Treatment options of branch varicosities
▪ Ambulatory stab phlebectomy.
▪ Transilluminated-powered phlebectomy (TIPP).
Technical considerations for TIPP
▪ Clusters of veins are encircled with a marker prior to placing the patient supine.
▪ Stab incisions are made circumferentially around the vein clusters.
▪ The illuminator and resector hand pieces are passed into the superficial subcutaneous tissues.
▪ Liberal use of tumescence anesthesia and stab incisions decrease postoperative bruising and hematoma.
▪ Compression therapy is applied immediately following TIPP.
TIPP has the advantage over stab phlebectomy of fewer incisions & quicker procedural time when treating large clusters of varicosities
▪ The learning curve must be overcome in order to achieve optimal results with TIPP.
▪ Once a mastery of TIPP is obtained, introducing TIPP into the office setting may be a possibility.
References
Papers of special note have been highlighted as:
▪▪ of considerable interest
- Eberhardt RT, Raffetto JD. Chronic venousinsufficiency. Circulation 111(18), 2398–2409(2005).
- Beebe-Dimmer JL, Pfeifer JR, Engle JS,Schottenfeld D. The epidemiology of chronicvenous insufficiency and varicose veins. Ann.Epidemiol. 15(3), 175–184 (2005).
- Eklof B, Rutherford RB, Bergan JJ et al.Revision of the CEAP classification forchronic venous disorders: consensusstatement. J. Vasc. Surg. 40(6), 1248–1252(2004).
- Lim CS, Davies AH. Pathogenesis of primaryvaricose veins. Br. J. Surg. 96(11), 1231–1242(2009).
- Carpentier PH, Maricq HR, Biro C,Poncot-Makinen CO, Franco A. Prevalence,risk factors, and clinical patterns of chronicvenous disorders of lower limbs: apopulation-based study in France. J. Vasc.Surg. 40(4), 650–659 (2004).
- Laurikka JO, Sisto T, Tarkka MR, AuvinenO, Hakama M. Risk indicators for varicoseveins in forty- to sixty-year-olds in theTampere varicose vein study. World J. Surg.26(6), 648–651 (2002).
- Scott TE, LaMorte WW, Gorin DR,Menzoian JO. Risk factors for chronicvenous insufficiency: a dual case-controlstudy. J. Vasc. Surg. 22(5), 622–628 (1995).
- Hirai M, Naiki K, Nakayama R. Prevalenceand risk factors of varicose veins in Japanesewomen. Angiology 41(3), 228–232 (1990).
- Brand FN, Dannenberg AL, Abbott RD,Kannel WB. The epidemiology of varicoseveins: the Framingham Study. Am. J. Prev.Med. 4(2), 96–101 (1988).
- Evans CJ, Fowkes FG, Ruckley CV, Lee AJ.Prevalence of varicose veins and chronicvenous insufficiency in men and women inthe general population: Edinburgh Vein Study. J. Epidemiol. Community Health53(3), 149–153 (1999).
- Dindelli M, Parazzini F, Basellini A,Rabaiotti E, Corsi G, Ferrari A. Risk factorsfor varicose disease before and duringpregnancy. Angiology 44(5), 361–367 (1993).
- Seidell JC, Bakx KC, Deurenberg P, van denHoogen HJ, Hautvast JG, Stijnen T.Overweight and chronic illness – a retrospectivecohort study, with a follow-up of 6–17 years, inmen and women of initially 20–50 years of age.J. Chronic Dis. 39(8), 585–593 (1986).
- Tuchsen F, Hannerz H, Burr H, Krause N.Prolonged standing at work andhospitalisation due to varicose veins: a 12 yearprospective study of the Danish population.Occup. Environ. Med. 62(12), 847–850(2005).
- Tuchsen F, Krause N, Hannerz H, Burr H,Kristensen TS. Standing at work and varicoseveins. Scand. J. Work Environ. Health 26(5),414–420 (2000).
- Gloviczki P, Comerota AJ, Dalsing MC et al.The care of patients with varicose veins andassociated chronic venous diseases: clinicalpractice guidelines of the Society forVascular Surgery and the American VenousForum. J. Vasc. Surg. 53(Suppl. 5), S2–S48(2011).
- Brar R, Nordon IM, Hinchliffe RJ, LoftusIM, Thompson MM. Surgical managementof varicose veins: meta-analysis.Vascular18(4), 205–220 (2010).
- Nordon IM, Hinchliffe RJ, Brar R et al.A prospective double-blind randomizedcontrolled trial of radiofrequency versus lasertreatment of the great saphenous vein inpatients with varicose veins. Ann. Surg.254(6), 876–881 (2011).
- Nesbitt C, Eifell RK, Coyne P, Badri H,Bhattacharya V, Stansby G. Endovenous ablation (radiofrequency and laser) and foamsclerotherapy versus conventional surgery forgreat saphenous vein varices. CochraneDatabase Syst. Rev. (10), CD005624 (2011).
- Carradice D, Mekako AI, Hatfield J, ChetterIC. Randomized clinical trial of concomitantor sequential phlebectomy after endovenouslaser therapy for varicose veins. Br. J. Surg.96(4), 369–375 (2009).
- Gale SS, Lee JN, Walsh ME,Wojnarowski DL, Comerota AJ.A randomized, controlled trial of endovenousthermal ablation using the 810‑nmwavelength laser and the ClosurePLUSradiofrequency ablation methods forsuperficial venous insufficiency of the greatsaphenous vein. J. Vasc. Surg. 52(3), 645–650(2010).
- Goode SD, Chowdhury A, Crockett M et al.Laser and radiofrequency ablation study(LARA study): a randomised studycomparing radiofrequency ablation andendovenous laser ablation (810 nm).Eur. J. Vasc. Endovasc. Surg. 40(2), 246–253(2010).
- Rasmussen LH, Lawaetz M, Bjoern L,Vennits B, Blemings A, Eklof B. Randomizedclinical trial comparing endovenous laserablation, radiofrequency ablation, foamsclerotherapy and surgical stripping for greatsaphenous varicose veins. Br. J. Surg. 98(8),1079–1087 (2011).
- Almeida JI, Kaufman J, Gockeritz O et al.Radiofrequency endovenous ClosureFASTversus laser ablation for the treatment of greatsaphenous reflux: a multicenter, singleblinded,randomized study (RECOVERYstudy). J. Vasc. Interv. Radiol. 20(6), 752–759(2009).
- Shepherd AC, Gohel MS, Brown LC,Metcalfe MJ, Hamish M, Davies AH.Randomized clinical trial of VNUSClosureFAST radiofrequency ablation versuslaser for varicose veins. Br. J. Surg. 97(6),810–818 (2010).
- Cheshire N, Elias SM, Keagy B et al. Poweredphlebectomy (TriVex) in treatment of varicoseveins. Ann. Vasc. Surg. 16(4), 488–494 (2002).
- Passman M. Transilluminated poweredphlebectomy in the treatment of varicoseveins. Vascular 15, 262–268 (2007).
- Scavee V. Transilluminated poweredphlebectomy: not enough advantages? Reviewof the literature. Eur. J. Vasc. Endovasc. Surg.31(3), 316–319 (2006).
- Chetter IC, Mylankal KJ, Hughes H,Fitridge R. Randomized clinical trialcomparing multiple stab incision phlebectomyand transilluminated powered phlebectomyfor varicose veins. Br. J. Surg. 93(2), 169–174(2006).
- Aremu MA, Mahendran B, Butcher W et al.Prospective randomized controlled trial:conventional versus powered phlebectomy.J. Vasc. Surg. 39(1), 88–94 (2004).
- Scavee V, Lesceu O, Theys S, Jamart J,Louagie Y, Schoevaerdts JC. Hookphlebectomy versus transilluminated poweredphlebectomy for varicose vein surgery: earlyresults. Eur. J. Vasc. Endovasc. Surg. 25(5),473–475 (2003).
- Shamiyeh A, Schrenk P, Huber E, Danis J,Wayand WU. Transilluminated poweredphlebectomy: advantages and disadvantagesof a new technique. Dermatol. Surg. 29(6),616–619 (2003).
- Spitz G. Transilluminated poweredphlebectomy in an office setting: proceduralconsiderations and clinical outcomes.J. Endovasc. Ther. 18(5), 734–738 (2011)
- Franz RW, Knapp ED. Transilluminatedpowered phlebectomy surgery for varicoseveins: a review of 339 consecutive patients.Ann. Vasc. Surg. 23(3), 303–309 (2009).
- Sam RC, Silverman SH, Bradbury AW. Nerveinjuries and varicose vein surgery. Eur. J. Vasc.Endovasc. Surg. 27(2), 113–120 (2004).
▪▪ Major document from the American Venous Forum and the Society for Vascular Surgery providing up-to-date guidelines.
▪▪ Excellent manuscript describing the technique of transilluminated-powered phlebectomy.
▪▪ Interesting study suggesting that transilluminated-powered phlebectomy, in certain circumstances, may be performed in an office setting.
▪▪ Largest published series on transilluminatedpowered phlebectomy to date of over 330 patients showing almost 90% of patients requiring just 20 or fewer incisions, and extremely high patient satisfaction ratings.