Review Article - Interventional Cardiology (2012) Volume 4, Issue 5
Vascular closure devices in percutaneous coronary and peripheral interventions: rationale and results
- Corresponding Author:
- Dushad Ram
Department of Psychiatry
JSS Medical College and Hospital MG Road Agrahara,
Mysore, Karnataka, India, 570004
Tel: 09008740965
Fax: 416-603-6919
E-mail: Arina.Bingeliene@uhn.ca
Abstract
Keywords
access site complications,arteriovenous fistula,coronary intervention,hematoma,hemostasis,peripheral intervention,pseudoaneurysm,vascular closure device
Introduction
Coronary artery disease is a major cause of morbidity and mortality worldwide. With the combination of continuously improving equipment and modern drug therapy, percutaneous coronary intervention (PCI) has evolved into an effective nonsurgical modality for treating patients with coronary artery disease. Ongoing technical advances have also allowed nonsurgical revascularization of other vascular beds including percutaneous peripheral interventions. With a rapid increase in percutaneous diagnostic and interventional procedures performed worldwide, hemostasis strategy has become a critical topic of interest and research [1]. Although infrequent, vascular access site complications affect morbidity, mortality, costs and length of hospital stay. This review will present the evidence gathered from various studies regarding the efficacy and safety of the use of vascular closure devices (VCDs) in PCIs and noncoronary interventions.
Vascular access complications
The common femoral artery (CFA) is the most common access site for cardiac catheterizations. The CFA extends from the external iliac artery as it courses alongside the medial aspect of the femoral head, midway between the anterior superior iliac spine and the pubic symphysis [2]. Access site complications in angiographic and angioplasty procedures involving femoral artery punctures have been reported in 1–9% of cases [3]. These complications range from simple hematomas to arterial thrombosis, pseudoaneurysm, embolization, arteriovenous fistula, retroperitoneal hemorrhage, arterial hemorrhage requiring transfusion and possible surgical repair. The risk of vascular complications associated with coronary and peripheral interventions has been shown to depend on patient characteristics and the type of procedure performed [4]. Patient characteristics associated with a low risk include male sex, younger age, preserved renal function and increased body size. Patients with known coagulopathy, peripheral artery disease, immunosuppression and renal dysfunction are considered to be at high risk with major vascular complications rates exceeding 3%. Diagnostic cardiac catheterizations are associated with a lower rate of vascular complications, with overall risk reported to be <1%. Patients undergoing nonemergent or elective PCI are considered to be at moderate risk for vascular complications with an overall reported rate of 1–3% [4,5]. Stent placement increases the risk of vascular complications as it necessitates vigorous anticoagulation [6]. While the femoral arterial puncture remains the most common access method for coronary angiography and PCIs, the radial artery approach is a safe and effective alternative. Transradial intervention has been associated with a reduced risk of vascular complications compared with femoral artery access, especially access site-related bleeding complication, leading to reduction in morbidity in PCI [7,8]. Multiple studies including ACCESS and CARAFE have reported lower rates of major access site complications associated with the radial artery approach [9,10].
VCDs are increasingly being utilized for access site management in percutaneous coronary and peripheral interventions. Many of these peripheral interventions (and more recently structural heart interventions) are performed in the treatment of elderly, high-risk patients with calcified vessels using large caliber sheaths [11]. It has been shown that the risk of vascular complications is highest with emergent PCIs and interventions necessitating sheath sizes greater than 8 French (F) [12]. Two additional factors that may affect the risk of vascular complications include device failure, as well as the learning curve, or the period of time required for the providers to become familiarized with the particular device and acquire the necessary skill and expertise. Bangalore et al. evaluated the frequency of VCD failure and the impact that VCD failure had on risk of vascular complications. They found that failure, defined as unsuccessful deployment or failure to achieve hemostasis, was associated with a significantly increased risk of major (1.9 vs 0.6%) and minor (6 vs 1.1%) complications [13].
Rationale for developing VCDs
Traditionally, hemostasis after PCIs was achieved with manual compression followed by 6 h of bed rest. Manual compression is labor intensive, time consuming and can be a significant source of patient discomfort. Over the years, VCDs have been developed that improve patient comfort compared with manual compression and reduce time-to-hemostasis and early ambulation [14]. These benefits translate into increased patient satisfaction and reduced hospital length of stay. While VCDs have been shown to improve patient comfort, promote early ambulation and decrease time-to-hemostasis, the evidence for the safety and efficacy of these devices is still controversial [15]. Multiple meta-analyses, prospective randomized and nonrandomized studies have shown conflicting results.
Overview of VCDs
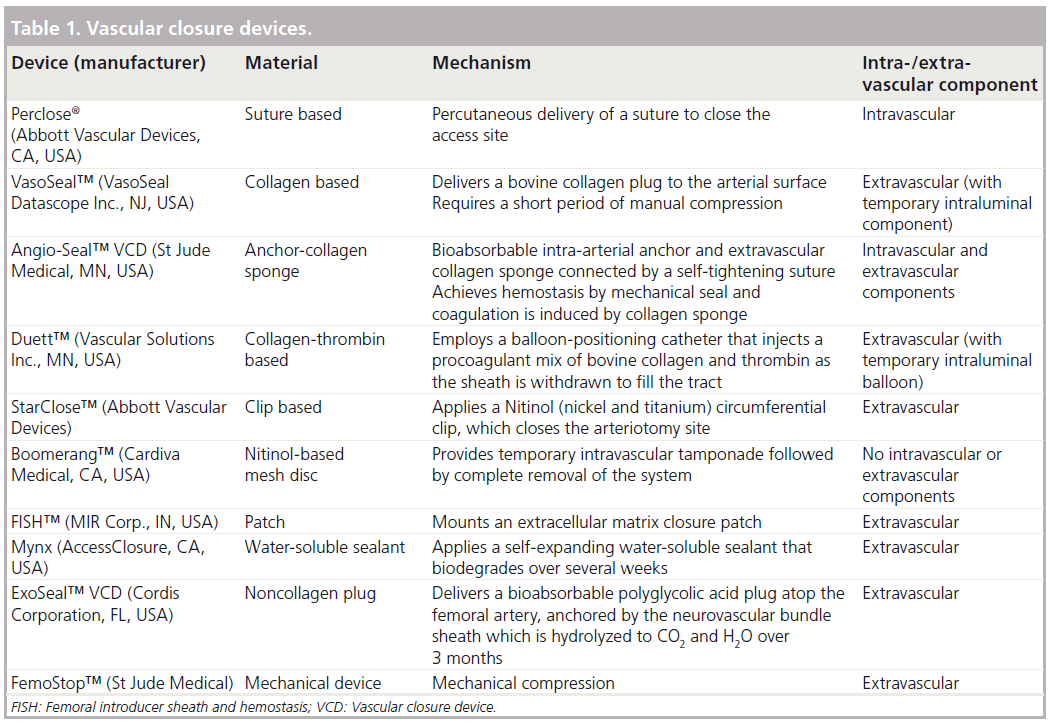
The development of the first vascular closure device in the 1990s provided a suitable alternative to the gold standard of manual compression to achieve hemostasis. Over the years, numerous advancements have been made and multiple devices are now available [16]. Initial devices such as the Perclose® (Abbott Vascular Devices, CA, USA) relied on percutaneous placement of sutures to close the arteriotomy site, while others such as VasoSeal™ (VasoSeal Datascope Inc., NJ, USA) and Angio-Seal™ (St Jude Medical, MN, USA) are based on the delivery of a collagen plug with or without an intravascular anchor system [17–19]. The VasoSeal device is no longer available in the USA. The Duett™ (Vascular Solutions Inc., MN, USA) VCD utilizes a balloon- positioning catheter to inject a procoagulant mixture of bovine microfibrillar collagen into the tract created by the sheath, thereby facilitating extravascular thrombosis in the perivascular tissue [20]. Numerous other devices have emerged more recently, which use alternative methods to achieve hemostasis. The femoral introducer sheath and hemostasis device (MIR Corp., IN, USA) employs an extracellular matrix closure patch [21]. The Mynx (AccessClosure, CA, USA) system deploys a self-expanding water-soluble sealant [22]. The ExoSeal™ (Cordis Corporation, FL, USA) delivers an extravascular noncollagen polyglycolic acid plug atop the femoral artery, anchored by the neurovascular bundle sheath [21,22]. An overview of the available closure devices is presented in Table 1.
Results
▪ Neutral or increased risk of vascular access complications
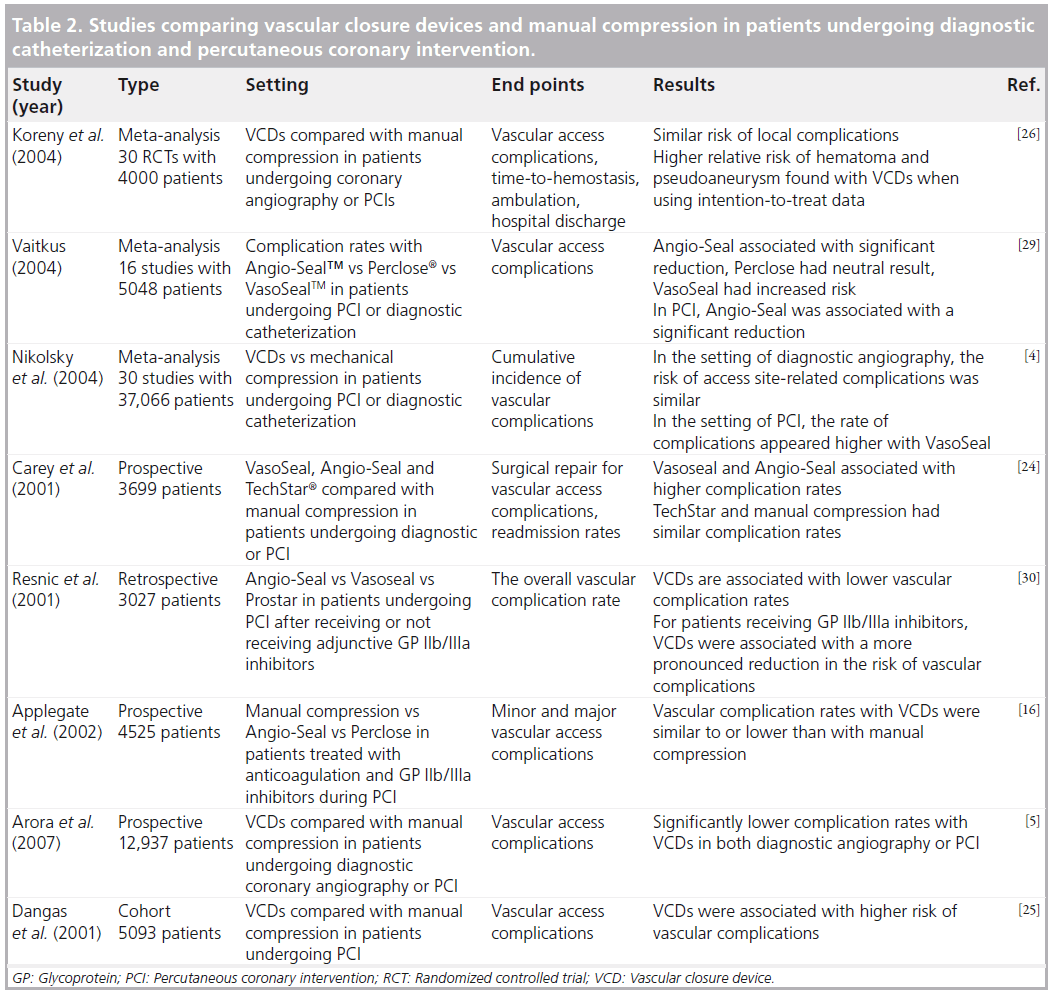
Of the 10 million percutaneous vascular procedures performed in the USA in 2007, VCDs were used in 30% of the cases [23]. Despite the progress in VCD technology and catheter-based deployment techniques, concerns still remain regarding their safety and efficacy (Table 2). Several large observational studies have attributed vascular access site complications after endovascular treatment to VCDs. One large prospective study by Carey et al. involving 3699 patients found VasoSeal and Angio-seal devices to be associated with higher rates of total complications compared with manual compression [24]. Femoral artery occlusion, a rare, serious, potentially limb threatening complication, was noted in five of the 742 patients with the Angio-Seal device. The study also raised the concern of increased risk of infection with VCDs, as groin infections were encountered in 0.3% of VCDs but were not seen in patients with manual groin compression [24]. One potential limitation of this study, which also plagued several of the preceding studies, relates to the learning curve, or the period of time required for the providers to become familiar with the particular device and acquire the necessary skill and expertise. A large cohort study published by Dangas et al., involving 5093 patients, focused on vascular complications following percutaneous intervention and found higher complication rates with VCDs compared with manual compression [25]. VCDs evaluated in the study included Angio- Seal, Duett, VasoSeal, Prostar® (Abbott Vascular Devices) and Perclose. In this study, hematomas were found to occur more frequently with the use of VCDs compared with manual compression and vascular surgical repair at the access site was required more often. A higher rate of significant bleeding (defined as hematocrit drop >15%), was also noted with VCDs compared with manual compression (5.2 vs 2.5%; p < 0.001) [25]. Another meta-analysis by Koreny et al. included 30 randomized controlled trials comparing the safety and efficacy of multiple different VCDs including Angio-Seal, VasoSeal, Duett, Perclose, TechStar® (Abbott Vascular Devices) or Prostar with manual compression in patients undergoing diagnostic coronary angiography or PCI [26]. This study found minimal evidence in support of the effectiveness of VCDs and reported a relative risk of groin hematoma of 1.14 and pseudoaneurysm at the puncture site of 1.19, further raising concerns regarding the safety and efficacy of VCDs [26]. Nikolsky et al. reviewed 30 randomized control trials and cohort studies comparing Angio-Seal, Vasoseal or Perclose with manual compression and found similar rates of vascular complications in patients undergoing diagnostic angiography [4]. However, in the setting of PCI, the rate of complications appeared to be higher with the VasoSeal device. In a retrospective analysis of the three generations of the Angio-Seal device, Applegate et al. found no significant differences between the three devices and manual compression [16]. Successful closure rates were similar for diagnostic and PCI procedures; the overall success rate was greater than 98%. The vascular complication rates were similar to those associated with manual compression [16].
▪ Reduced risk of vascular access complications
Interestingly, Chevalier et al. conducted a multicenter, randomized trial comparing the efficacy of Angio-Seal with manual compression in reducing access site complications in high-risk patients undergoing PCI [27]. The study included 612 patients from 11 different centers and reported reduction in duration of bed rest and time-to-hemostasis in the Angio- Seal group, but found length of hospital stay to be similar in both groups. The primary end point was the composite incidence of at least one of seven access site complications, including surgical repair, bleeding requiring transfusion or prolonged compression, infection, hematoma, deep venous thrombosis, arteriovenous fistula and pseudoaneurysm. The composite incidence of vascular complications was found to be significantly lower in the AngioSeal group compared with manual compression (5.9 vs 18%), making this the first multicenter randomized trial to show a significant reduction in complication rates in high-risk patients [27]. In an attempt to determine whether VCDs impact major access site bleeding (ASB) in patients with acute coronary syndromes undergoing early invasive management via femoral artery access, Sanborn et al. conducted a post hoc subgroup analysis of the prospective, multicenter, randomized ACUITY trial. Major ASB was defined as bleeding requiring interventional or surgical correction, hematoma greater than or equal to 5 cm, retroperitoneal bleeding, or hemoglobin drop greater than or equal to 3 g/dl with echymosis or hematoma <5 cm, oozing blood, or bleeding longer than 30 min at the access site. This study found the rates of major femoral ASB to be significantly lower with VCD use compared with manual compression (2.5 vs 3.3%). Interestingly, the lowest rate of ASB (<1%) was found in patients who were treated with bivalirudin monotherapy and a VCD, suggesting the combined use of bivalirudin and VCD may reduce major ASB in this patient population [28]. Many of the randomized control trials evaluating complication rates are limited by their small sample size, making them underpowered to detect clinically significant differences. In an attempt to address this limiting factor, several meta-analyses were performed. One such study by Vaitkus, analyzed 16 studies enrolling 5048 patients and found significant variation in risk reduction for specific devices compared with manual compression. Angio- Seal was associated with a significant reduction in risk, Perclose had a neutral result and VasoSeal was found to have an increased risk of complications [29]. Several other large studies, including a large prospective study by Arora et al. involving 12,937 patients have suggested the superiority of these devices compared with manual compression. This study evaluated manual compression versus Angio-Seal, Perclose, VasoSeal or Duett for access site closure in patients undergoing diagnostic angiography or PCI [5]. Resnic et al. performed a subgroup analysis of patients receiving glycoprotein IIb/IIIa antagonists in a retrospective study and reported a more pronounced reduction in the risk of vascular complications with the use of VCDs. VCDs evaluated in the study included Angio-Seal, VasoSeal and Prostar [30]. Another randomized, multicenter trial involving 435 patients undergoing cardiac catheterization or angioplasty in eight participating centers compared Angio-Seal versus manual compression [14]. Patients randomized to the Angio-Seal group were reported to have lower bleeding complication rates compared with those randomized to the manual compression group. Interestingly, the mean time-to-hemostasis was doubled in the manual compression group with the use of heparin and the overall complication rate increased from 6 to 27%. No significant difference was noted in the complication rate of patients receiving heparin in the Angio-Seal group [14]. These studies suggest that the use of VCDs may affect risk of vascular complications when anticoagulant/antiplatelet therapy is used.
▪ Closure devices in patients undergoing noncoronary interventions
Ben-Dor et al. compared the safety and efficacy of the collagen-mediated device with suture-mediated techniques and manual compression for access site management following balloon aortic valvuloplasty [11]. Their data suggested improved safety of collagen-based closure devices and suture-mediated closure devices over manual compression, with collagen-based closure devices having lower failure rates than suturemediated closure devices on further analysis [11]. Jahnke et al. reported safety and efficacy of total percutaneous access closure for endovascular aortic aneurysm repair with a suture-mediated
Perclose technique in a case–control study [31]. A dual 6-F-Perclose device technique was found to have fewer late groin complications and scar tissue formation compared with femoral cut down. A nonrandomized prospective study performed by Ratnam et al. compared complication rates following CFA puncture using Angio-Seal, StarClose™ (Abbott Vascular Devices) or manual compression [32]. Data were collected from 429 CFA punctures over the course of 1 year. Interestingly, the study found a higher rate of failed initial hemostasis with the StarClose device. The study also demonstrated a significantly increased risk of major complications in women with peripheral vascular disease. However, no significant difference in major complication rates was found amongst the devices. Overall, the study concluded that Angio-Seal and StarClose were safe alternatives to manual compression for use in peripheral vascular procedures [32]. Another study by Upponi et al., compared the use of Angio-Seal and manual compression following peripheral vascular diagnostic and interventional procedures in a randomized control trial involving 100 patients [33]. The study concluded that there were no significant differences in complication rates after 1 week. However, there was a significant difference in time-to-hemostasis between the groups, with the mean time for the Angio-Seal group recorded as 2 min versus 10.6 min in the compression group [33].
▪ Closure devices for brachial approach
While VCDs are currently indicated for use in closure of femoral artery puncture sites, recent studies have been carried out that raise the question of whether or not these devices would provide similar benefits when using a brachial approach. The brachial artery, being a smaller caliber vessel, raises the concern that use of suture or collagen-based VCDs, such as Perclose or Angio-Seal, may lead to vessel stenosis.
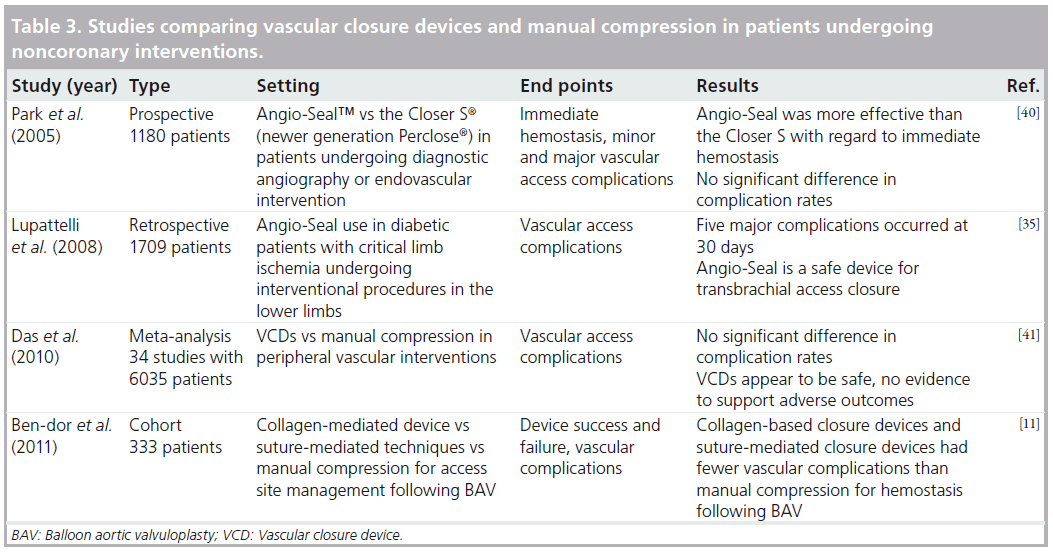
A recent case report by Cirillo et al. was the first report describing the use of the Boomerang™ device (Cardiva Medical, CA, USA) to attain hemostasis following brachial artery puncture after emergent percutaneous transluminal coronary angioplasty in humans. The study found no complications, suggesting that this device may be a useful alternative to manual hemostasis for closure of brachial artery access [34]. Another study by Lupattelli et al. evaluated vascular complications following Angio-Seal closure of brachial artery puncture site [35]. The study population included 238 diabetic patients undergoing interventional procedures in the lower limbs. At 30 days, the Angio-Seal group had major complication rate of 3.1% and a minor complication rate of 7.5%. Major complications included two puncture site hematomas larger than 4 cm, two brachial artery occlusions and one pseudoaneurysm. Minor complications included three hematomas less than 4 cm, three cases of oozing from the access site and six patients who experienced minor pain. Overall, this study concluded that Angio-Seal could be safely used for arterial closure using brachial access in diabetic patients undergoing lower limb procedures for critical limb ischemia [35]. In a similar prospective study by Belenky et al., the efficacy and safety of Angio-Seal for hemostasis of distal brachial artery site puncture was evaluated. The study population included 64 patients and found a deployment success rate of 100% with no major complications [36]. Puggioni et al. performed a retrospective study that evaluated complication rates with the use of StarClose following brachial artery closure in 29 patients [37]. The device was deployed with successful achievement of hemostasis in 27 of the 29 patients. One patient developed a large hematoma, which was managed with prolonged manual compression. One patient lost forearm pulses, requiring emergent surgical intervention, which revealed incorrect clip release in the perivascular tissues. After a mean follow-up of 7.5 months, all patients were found to have palpable brachial and radial pulses with no signs of infection, distal embolization or neurological deficits. The study concluded that StarClose was a safe and effective method of achieving hemostasis following interventional procedures via brachial artery access [37]. Table 3 lists major studies that evaluated VCDs in patients undergoing peripheral interventions and/or in patients with vascular access sites other than the femoral artery.
Discussion
Although VCDs have been proven to reduce timeto- hemostasis and time-to-ambulation compared with manual compression, there is no conclusive evidence that they reduce the rate of complications. One potential contributing factor probably relates to the learning curve, or the period of time required for the providers to become familiarized with the particular device and acquire the necessary skill and expertise. More experienced centers have reported low failure rates [38]. Proper femoral angiography is a prerequisite for reducing access site complications and using fluoroscopy to guide puncture of the anterior wall of the CFA directly overlying the femoral head has been shown to be beneficial in reducing complications [39]. The importance of meticulous patient selection when using VCDs cannot be overemphasized. Finally, all VCDs are subject to failure, and manual and/ or mechanical pressure using a compression assist device, such as the FemoStop™ (St Jude Medical), may be required if bleeding occurs despite their use. As VCDs become more mainstream and operator experience increases, more data will become available that will help define their role in hemostasis strategy. Data from multiple large, multicenter studies and meta-analysis have supported VCDs as a safe and effective alternative to manual compression. However, the evidence with regard to their comparative efficacy remains controversial. Adequately powered randomized controlled trials are required to further elucidate the efficacy of VCDs and their role in hemostasis strategy.
Future perspective
Since they first emerged in the mid-1990s, VCDs have undergone substantial technological advances by incorporating a variety of different materials and methods of deployment targeted at improving efficacy and safety. Although it is clear that VCDs improve patient comfort and reduce time-to-ambulation over manual compression, data thus far are largely from observational studies, with the available randomized studies lacking power to conclude significant or generalizable results. Future studies involving large-scale, randomized controlled trials are necessary to further explore the benefits and risks offered by VCDs. The importance of developing standardized end points and a system that can be used universally to more accurately compare and contrast these devices should be investigated.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
▪▪ Vascular access site complications following percutaneous cardiovascular interventions remain a major cause of morbidity and mortality.
Vascular access complications
▪▪ Complications include retroperitoneal hemorrhage, pseudoaneurysm formation, hematoma, arteriovenous fistula and leg ischemia.
Rationale for developing vascular closure devices
▪▪ Vascular closure devices have been shown to improve patient comfort, promote early ambulation and decrease time-to-hemostasis. However, the evidence regarding the safety and efficacy of these devices is not conclusive.
Discussion
▪▪ Adequately powered randomized controlled trials are required to further elucidate the efficacy of vascular closure devices and their role in reducing access site complications.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Irani F, Kumar S, Colyer WR Jr. Common femoral artery access techniques: a review. J. Cardiovasc. Med. 10(7), 517–522 (2009).
- Schnyder G, Sawhney N, Whisenant B, Tsimikas S, Turi ZG. Common femoral artery anatomy is influenced by demographics and comorbidity: implications for cardiac and peripheral invasive studies. Catheter. Cardiovasc. Interv. 53(3), 289–295 (2001).
- Muller DW, Shamir KJ, Ellis SG, Topol EJ. Peripheral vascular complications after conventional and complex percutaneous coronary interventional procedures. Am. J. Cardiol. 69(1), 63–68 (1992).
- Nikolsky E, Mehran R, Halkin A et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J. Am. Coll. Cardiol. 44(6), 1200–1209 (2004).
- Arora N, Matheny ME, Sepke C, Resnic FS. A propensity analysis of the risk of vascular complications after cardiac catheterization procedures with the use of vascular closure devices. Am. Heart J. 153(4), 606–611 (2007).
- de Jaegere PP, de Feyter PJ, van der Giessen WJ, Serruys PW. Endovascular stents: preliminary clinical results and future developments. Clin. Cardiol. 16(5), 369–378 (1993).
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am. Heart J. 157(1), 132–140 (2009).
- Agostoni P, Biondi-Zoccai GG, de Benedictis ML et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: systematic overview and meta-analysis of randomized trials. J. Am. Coll. Cardiol. 44(2), 349–356 (2004).
- Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J. Am. Coll. Cardiol. 29(6), 1269–1275 (1997).
- Louvard Y, Lefevre T, Allain A, Morice M. Coronary angiography through the radial or the femoral approach: the CARAFE study. Catheter. Cardiovasc. Interv. 52(2), 181–187(2001).
- Ben-Dor I, Looser P, Bernardo N et al. Comparison of closure strategies after balloon aortic valvuloplasty: suture mediated versus collagen based versus manual. Catheter. Cardiovasc. Interv. 78(1), 119–124 (2011).
- Patel MR, Jneid H, Derdeyn CP et al. Arteriotomy closure devices for cardiovascular procedures: a scientific statement from the American Heart Association. Circulation 122(18), 1882–1893 (2010).
- Bangalore S, Arora N, Resnic FS. Vascular closure device failure: frequency and implications: a propensity-matched analysis. Circ. Cardiovasc. Interv. 2(6), 549–556(2009).
- Kussmaul WG 3rd, Buchbinder M, Whitlow PL et al. Rapid arterial hemostasis and decreased access site complications after cardiac catheterization and angioplasty: results of a randomized trial of a novel hemostatic device. J. Am. Coll. Cardiol. 25(7), 1685–1692 (1995).
- Prada-Delgado O, Estevez-Loureiro R, Calvino-Santos R et al. Safety and efficacy of femoral vascular closure devices in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction. Am. Heart J. 161(6), 1207–1213 (2011).
- Applegate RJ, Grabarczyk MA, Little WC et al. Vascular closure devices in patientstreated with anticoagulation and IIb/IIIa receptor inhibitors during percutaneous revascularization. J. Am. Coll. Cardiol. 40(1), 78–83 (2002).
- Chamberlin JR, Lardi AB, McKeever LS et al. Use of vascular sealing devices (VasoSeal and Perclose) versus assisted manual compression (Femostop) in transcatheter coronary interventions requiring abciximab (ReoPro). Catheter. Cardiovasc. Interv. 47(2), 143–147;discussion 148 (1999).
- Gwechenberger M, Katzenschlager R, Heinz G, Gottsauner-Wolf M, Probst P. Use of a collagen plug versus manual compression for sealing arterial puncture site after cardiac catheterization. Angiology 48(2), 121–126 (1997).
- Veasey RA, Large JK, Silberbauer J et al. A randomised controlled trial comparing StarClose and AngioSeal vascular closure devices in a district general hospital – the SCOAST study. Int. J. Clin. Pract. 62(6), 912–918 (2008).
- Hong K, Liapi E, Georgiades CS, Geschwind JF. Case-controlled comparison of a percutaneous collagen arteriotomy closure device versus manual compression after liver chemoembolization. J. Vasc. Interv. Radiol.16(3), 339–345 (2005).
- Bavry AA, Raymond RE, Bhatt DL et al. Efficacy of a novel procedure sheath and closure device during diagnostic catheterization: the multicenter randomized clinical trial of the FISH device. J. Invasive Cardiol. 20(4), 152–156 (2008).
- Fargen KM, Velat GJ, Lawson MF et al. Occurrence of angiographic femoral artery complications after vascular closure with Mynx and AngioSeal. J. Neurointerv. Surg. (2012).
- Wong SC, Bachinsky W, Cambier P et al. A randomized comparison of a novel bioabsorbable vascular closure device versus manual compression in the achievement of hemostasis after percutaneous femoral procedures: the ECLIPSE (Ensure’s Vascular Closure Device Speeds Hemostasis Trial). JACC Cardiovasc. Interv. 2(8), 785–793(2009).
- Carey D, Martin JR, Moore CA, Valentine MC, Nygaard TW. Complications of femoral artery closure devices. Catheter. Cardiovasc. Interv. 52(1), 3–7; discussion 8 (2001).
- Dangas G, Mehran R, Kokolis S et al. Vascular complications after percutaneous coronary interventions following hemostasis with manual compression versus arteriotomy closure devices. J. Am. Coll. Cardiol. 38(3), 638–641 (2001).
- Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA 291(3), 350–357 (2004).
- Chevalier B, Lancelin B, Koning R et al. Effect of a closure device on complication rates in high-local-risk patients: results of a randomized multicenter trial. Catheter. Cardiovasc. Interv. 58(3), 285–291 (2003).
- Sanborn TA, Ebrahimi R, Manoukian SV et al. Impact of femoral vascular closuredevices and antithrombotic therapy on access site bleeding in acute coronary syndromes: The Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circ. Cardiovasc. Interv. 3(1), 57–62 (2010).
- Vaitkus PT. A meta-analysis of percutaneous vascular closure devices after diagnostic catheterization and percutaneous coronary intervention. J. Invasive Cardiol. 16(5), 243–246 (2004).
- Resnic FS, Blake GJ, Ohno-Machado L, Selwyn AP, Popma JJ, Rogers C. Vascular closure devices and the risk of vascular complications after percutaneous coronary intervention in patients receiving glycoprotein IIb-IIIa inhibitors. Am. J. Cardiol. 88(5), 493–496 (2001).
- Jahnke T, Schafer JP, Charalambous N et al. Total percutaneous endovascular aneurysm repair with the dual 6-F Perclose-AT preclosing technique: a case-control study.J. Vasc. Interv. Radiol. 20(10), 1292–1298 (2009).
- Ratnam LA, Raja J, Munneke GJ, Morgan RA, Belli AM. Prospective nonrandomized trial of manual compression and Angio-Seal and Starclose arterial closure devices in common femoral punctures. Cardiovasc. Intervent. Radiol. 30(2), 182–188 (2007).
- Upponi SS, Ganeshan AG, Warakaulle DR, Phillips-Hughes J, Boardman P, Uberoi R. Angioseal versus manual compression for haemostasis following peripheral vascular diagnostic and interventional procedures – a randomized controlled trial. Eur. J. Radiol. 61(2), 332–334 (2007).
- Cirillo P, Petrillo G, D’Ascoli GL, Piscione F, Chiariello M. Successful use of the Cardiva Boomerang vascular closure device to close a brachial artery puncture site after emergency PTCA. Heart Vessels 25(6), 565–568 (2010).
- Lupattelli T, Clerissi J, Clerici G et al.The efficacy and safety of closure of brachial access using the AngioSeal closure device: experience with 161 interventions in diabetic patients with critical limb ischemia. J. Vasc. Surg. 47(4), 782–788 (2008).
- Belenky A, Aranovich D, Greif F, Bachar G, Bartal G, Atar E. Use of a collagen-based device for closure of low brachial artery punctures. Cardiovasc. Intervent. Radiol.30(2), 273–275 (2007).
- Puggioni A, Boesmans E, Deloose K, Peeters P, Bosiers M. Use of StarClose for brachial artery closure after percutaneous endovascular interventions. Vascular 16(2), 85–90 (2008).
- Mauro M, Thomson K, Venbrux A. Endovascular management of patients with chronic femoropopliteal disease. In: Image Guided Intervention (Volume 1). SaundersElsevier, PA, USA, 493–504 (2008).
- Lombardo A, van den Berg J. Preventing vascular access site complications during interventional procedures. Interv. Cardiol. 2(6), 829 (2010).
- Park Y, Roh HG, Choo SW et al. Prospective comparison of collagen plug (Angio-Seal) and suture-mediated (the Closer S) closure devices at femoral access sites. Korean J. Radiol. 6(4), 248–255 (2005).
- Das R, Ahmed K, Athanasiou T, Morgan RA, Belli AM. Arterial closure devices versus manual compression for femoral haemostasis in interventional radiological procedures: a systematic review and meta-analysis.Cardiovasc. Intervent. Radiol. 34, 723–738(2010).
▪▪ Comprehensive review of available data on the safety and efficacy of vascular closure devices.
▪ Meta-analysis of published data on closure devices versus manual compression.