Research Article - Neuropsychiatry (2019) Volume 9, Issue 2
Ventilatory Support Outcomes in Amyotrophic Lateral Sclerosis (ALS) Patients
- *Corresponding Author:
- Bebiana Conde
Institute for Research and Innovation in Health (i3S)
University of Porto, Rua Alfredo Allen, 4200-135 Porto, Portugal
Abstract
Background
Amyotrophic Lateral Sclerosis (ALS) represents the most common and severe motor neuron disease, with inevitable respiratory failure development. Ventilatory support (VS) has shown a valuable prognostic impact, even in bulbar-onset ALS. Thus, VS outcomes related to functional and phenotypic factors were analyzed in a cohort of ALS patients.
Methods and Findings
A prospective study was conducted in 81 patients with confirmed or probable ALS diagnosis, sent to a pulmonology clinic. From 81 patients enrolled, 11 dropped out, being only considered 70 patients (mean age 66.6 ± 11.3 years, 64.3% males, 52.9% ALS bulbar-onset) for analysis. During follow-up, VS was established in 50 patients (in 48 noninvasive ventilation). A good adherence was seen in 39 patients, with residual nocturnal events only observed in 10 patients. Regarding VS initiation criteria, 24 patients were eligible by functional criteria, 14 by nocturnal hypoventilation and 12 by daytime hypercapnia. After 3-6 months VS start, there was functional improvement in 17 patients. Survival after VS was 26.3 months, being higher in spinal-onset than in bulbar-onset ALS patients (p=0.012), and was even more evident in adherent spinal-onset ALS patients (p=0.022).
Conclusions
VS had a marked survival impact, leading to functional improvement, mainly when started by nocturnal hypoventilation criteria.
Keywords
Ventilatory support, Amyotrophic lateral sclerosis, Survival, Respiratory function
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a rare, progressive neurodegenerative and fatal disease. In Europe and United States of America the incidence is of 1-2/100.000 and prevalence of 3-5/100.000 inhabitants. Mean survival time is of 3-5 years and less than 20% live beyond 4 years [1]. Also, it has been reported that the time from the first ALS symptom to diagnosis is approximately 1 year [1]. Indeed, ALS represents the most common and severe motor neuron disease, with upper and lower motor neuron involvement, whose diagnosis was defined by El Escorial criteria [2]. Nevertheless, Awaji criteria may be more sensitive, namely in bulbar-onset ALS [3,4].
Based on body region involvement, there are different ALS phenotypes described: bulbar and pseudobulbar palsy and limb regional variants [1,5]. Nonetheless, this disease inevitable progresses to respiratory failure, but the effective VS use has shown a valuable prognostic impact [6,7]. According to the American Academy of Neurology (AAN) guidelines [8], the presence of hypoventilation symptoms (e.g. orthopnea), a Maximal Inspiratory Pressure (PImax)<60 cm H2O/Sniff Nasal Inspiratory Pressure (SNIP)<40 cm H2O, nocturnal desaturation, or a Forced Vital Capacity (FVC)<50%, constitute a VS indication in ALS patients. Furthermore, when cough is inefficient and Cough Peak Flow (CPF)<270/min, cough assistance must be initiated [8]. However, the literature data has shown that nocturnal hypoventilation is an adequate VS initiation criterion, even before diurnal hypercapnia, with better outcomes. Orlikowski et al. [9] showed that the “Ward” hypoventilation definition (maximum nocturnal TcCO2 ≥49 mmHg) comprises an effective and accurate VS criterion [9–11]. However, other hypoventilation definitions have also been proposed. For example, Simonds [12] considered a TcCO2>50 mmHg for VS initiation in neuromuscular diseases, while the American Academy for Sleep Medicine (AASM) established a TcCO2>55 mmHg for a period of more than 10 min or an increase in TcCO2>10 mmHg, when compared with awake supine value (TcCO2>50 mmHg) for a period exceeding 10 min [13].
Besides to the above described aspects, there are some factors related to bad prognosis in ALS patients, like bulbar and pseudobulbar onset, upper motor neuron compromise, VS failure and poor nutritional status [6,7,14]. One of the reasons for that seems to be due to swallowing, breathing and upper airways protection compromise, leading to respiratory failure and even death [1,2]. Moreover, respiratory muscle strength has also been proposed as a predictive biomarker for survival or even for ventilator-free survival in ALS patients, including PImax, SNIP, Vital Capacity as well as trans-diaphragmatic pressures [15]. However, even in face to a poor prognosis and progressive deterioration status, the effective VS use, coupled with Riluzole treatment, nutritional support and moderate intensity exercise have exerted a good prognostic impact in these patients [1,16–19].
There are some studies in literature describing the impact of different therapeutic approaches, namely using VS. For example, Sancho et al. [6] assessing VS prognostic impact in ALS ventilated patients found that, among the 120 patients intended to treat, VS conferred a longer survival (18.5 vs. 3 months), even in those with bulbaronset (13 vs. 3 months). On the other hand, Farrero et al. [20] observed a better survival only in non-bulbar ALS patients. But, to the authors’ knowledge, none of these studies analyzed the VS impact in overall survival time, evolution of respiratory function and admissions for respiratory exacerbation, in different groups of ALS patients, by different VS initiation criteria (e.g. lung function compromise, hypoventilation criteria or diurnal hypercapnia).
In this sense, based on the above highlighted aspects, we aimed to analyze the clinical outcomes in a cohort of ALS patients, and to identify either functional or phenotypic factors related with better outcomes.
Materials and Methods
▪ Participants
Eighty-one patients were consecutively enrolled in this study, conducted from January 2009 to January 2018, selecting patients with confirmed or probable ALS diagnosis made by Neurology department, according to El Escorial criteria, being immediately sent to the pulmonology department for functional evaluation and/or VS initiation.
During the quarterly follow-up, 11 patients dropped out, being only considered 70 ALS patients for further analysis. All of the patients with VS initiation criteria, according to the AAN guidelines [8] were allocated into three different groups based on the three formal criteria for VS initiation in ALS patients (i.e. functional changes, evidence of nocturnal hypoventilation and presence of daytime hypercapnia) [8,13].
This study had local Ethics Committee (Centro Hospitalar Trás-os-Montes e Alto Douro, Vila Real, Portugal) approval, and informed consent was obtained from all patients.
▪ Procedures
Every 3 to 6 months, recruited patients were asked to perform respiratory functional tests. Cough Peak Flow (CPF) was measured using Mini-Wright™ Peak Flow Meter (Clement Clarke International, England), as previously described by Winck et al. [21] and Suárez et al. [22]. Peak Expiratory Flow (PEF) was also determined using Mini-Wright Peak Flow Meter, and the corresponding PEF/CPF ratio was calculated.
Forced Vital Capacity (FVC), seated and supine, was measured using MicroLab™ Spirometer (CareFusion, USA), and we only choose the lowest value between them to increase FVC sensitivity [23,24]. Maximal Inspiratory (PImax) and Maximal Expiratory (PEmax) Pressures were determined by MicroRPM™ (CareFusion, USA). These values were determined according to the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines [25,26].
In the presence of hypoventilation symptoms without functional compromise, nocturnal polysomnographic evaluation was performed using the Alice 5® (Philips Respironics, USA) coupled to the TCM 400 monitor® (Radiometer, Denmark) for TcCO2 determination, according to AASM guidelines [13], in order to confirm or exclude nocturnal hypoventilation.
When ALS patients evidenced functional impairment according to the AAN guidelines [8], nocturnal hypoventilation according to the ASSM criteria [13] or diurnal hypercapnia (PaCO2>45 mmHg), VS initiation was proposed. If CPF<270 L/min, cough assistance was started. A suitable VS equipment was selected according to ventilatory dependency. For that, we mostly used Stellar™ 150 (ResMed, Australia), Astral™ 150 (ResMed, Australia) and Trilogy 100® (Philips Respironics, USA) equipment. The interfaces and VS modes were chosen for the best comfort and efficiency. Pressures titrations were performed during the day and/or night according to international recommendations [27], as sleep-disordered breathing may coexist in ALS patients [28]. Cough assistance was started as manually assisted cough, being only replaced by mechanically assisted one (using a Cough Assist E70®, Philips Respironics, USA), when the former was no longer able to achieve CPF>270 L/min [8].
▪ Statistical analyses
Categorical variables were described as absolute values (N) and relative frequencies, while continuous variables as mean and standard deviation (SD), or median, minimum and maximum values, when appropriate. Comparisons among two subgroups were performed with independent samples T-tests, whereas comparisons among three or more groups with one-way analysis of variance (ANOVA). Survival between different ALS phenotypes was assessed by Kaplan-Meier actuarial curve analysis. All data were analyzed using Statistical Package for the Social Sciences (SPSS, IBM Corp., USA)) software, version 25.0, with alpha set at 0.05.
Results
From the 70 ALS patients enrolled, with a mean age of 66.6 ± 11.3 years, 45 (64.3%) were males and 37 (52.9%) had ALS bulbar-onset. Cognitive compromise was observed in only 6 (8.6%) patients. At admission to respiratory evaluation, 43 (61.4%) ALS patients had hypoventilation symptoms and 44 (62.9%) had bulbar dysfunction symptoms (Table 1).
| Characteristics | Spinal (N=33) | Bulbar (N=37) | Total (N=70) | p Value |
|---|---|---|---|---|
| Age (years) | 63.2 ± 10.5 | 69.7 ± 11.2 | 66.6 ± 11.3 | 0.016 |
| Gender (N) | 0.000 | |||
| Female | 4 | 21 | 25 | |
| Male | 29 | 16 | 45 | |
| Clinical symptoms (N) | ||||
| Cognitive changes | 2 | 4 | 6 | 0.479 |
| Hypoventilation | 19 | 24 | 43 | 0.532 |
| Bulbar dysfunction | 9 | 35 | 44 | 0.000 |
| Hypercapnia (>45) | 8 | 11 | 19 | 0.606 |
| Lung function | ||||
| FVC (%) | 87.7 ± 21.4 | 62.2 ± 32.4 | 73.4 ± 30.7 | 0.000 |
| PEmax (cm H2O) | 72.4 ± 45.1 | 42.9 ± 36.8 | 55.1 ± 42.6 | 0.008 |
| PImax (cm H2O) | 57.1 ± 29.3 | 29.5 ± 24.8 | 40.7 ± 29.8 | 0.000 |
| PEF (L/min) | 294.1 ± 95.3 | 165.5 ± 113.6 | 226.0 ± 122.9 | 0.000 |
| CPF (L/min) | 262.0 ± 100.5 | 155.0 ± 132.0 | 205.4 ± 129.1 | 0.000 |
| VS and cough assistance characteristics | Spinal | Bulbar | Total | p Value |
| Ventilated patients (N) | 21 | 29 | 50 | 0.173 |
| Time from diagnosis VS (months) | 17.3 ± 19.0 | 11.0 ± 12.2 | 13.7 ± 15.6 | 0.162 |
| Reason for ventilating (N) | 0.049 | |||
| Hypercapnia | 6 | 6 | 12 | |
| Nocturnal Hypoventilation | 9 | 5 | 14 | |
| Functional | 6 | 18 | 24 | |
| FVC | 66.2 ± 20.6 | 52.2 ± 29.4 | 58.4 ± 26.6 | 0.080 |
| PImax (cmH2O) | 44.2 ± 27.8 | 26.1 ± 21.0 | 33.9 ± 25.5 | 0.018 |
| CPF (L/min) | 223.5 ± 109.0 | 120.8 ± 117.1 | 166.4 ± 123.6 | 0.004 |
| Adherence (N) | 0.906 | |||
| Good adherence (>4 h) | 17 | 22 | 39 | |
| Bad adherence (<4 h) | 5 | 7 | 12 | |
| Residual AHI uncorrected (N) | 0.089 | |||
| Yes | 2 | 8 | 10 | |
| No | 19 | 19 | 38 | |
| Cough supported patients (N) | 19 | 33 | 52 | 0.003 |
| Time from diagnosis (months) | 24.2 ± 22.6 | 9.7 ± 10.7 | 15.0 ± 17.4 | 0.015 |
| CPF in cough support (L/min) | 152.9 ± 84.3 | 100.9 ± 77.9 | 119.0 ± 83.2 | 0.036 |
| ABB: AHI: Apnea-Hypopnea Index; CPF: Cough Peak Flow; FVC: Forced Vital Capacity; PEF: Peak Expiratory Flow; PEmax: Maximal Expiratory Pressure; PImax: Maximal Inspiratory Pressure; VS: Ventilatory Support | ||||
Table 1: Patients demographics and clinical characteristics at study baseline and at time of ventilatory and cough support prescription.
On average, this evaluation was done 3 months after diagnosis, and the diagnosis was confirmed 13.7 months after the onset of symptoms. There was a mean follow-up time of 19 months in bulbar-onset and 32 months in spinal-onset ALS patients.
Regarding lung and respiratory muscle function assessment, ALS studied patients presented, at enrolment: mean FVC, PImax and PEmax values of, respectively, 73.4 ± 30.7%, 40.7 ± 29.8 cm H2O, and 55.1 ± 42.6 cm H2O, and cough mechanics: CPF=205.4 ± 129.1 L/min. Statistically significant differences were found on these parameters between bulbar-onset and spinal-onset ALS patients (Table 1).
During follow-up, VS was established in 50 (71.4%) patients, in almost all (96%) noninvasive ventilation, started on average 13.7 months (minimum 0 and maximum 67) after diagnosis, but sooner and with worst lung function in bulbar-onset ALS patients. A good adherence (defined as VS use of more than 4 h/ day or 120 h/month, and more than 70% of the days) was found in 39 (55.7%) patients, with residual obstructive events (Apnea-Hypopnea Index-AHI>5/h from the ventilator software) only occurring in 10 (14.9%) patients. VS was used on average 8 h/day, and 14 ALS patients were VS-dependent, with 24 h of use. In case of noninvasive VS dependence (16-24 h/daily), different kinds of interfaces were used (i.e. oronasal in nocturnal period and nasal or mouth piece during the diurnal period, when possible). Cough assistance was started in 52 (74.3%) patients, on average 15 months (minimum 1 and maximum 74) after diagnosis, also sooner and with worst CPF in bulbar-onset ALS patients, with statistically significant differences (Table 1). Among patients in whom VS was started, in 24 of them it was due to functional criteria, in 14 by nocturnal hypoventilation criteria and in 12 by daytime hypercapnia.
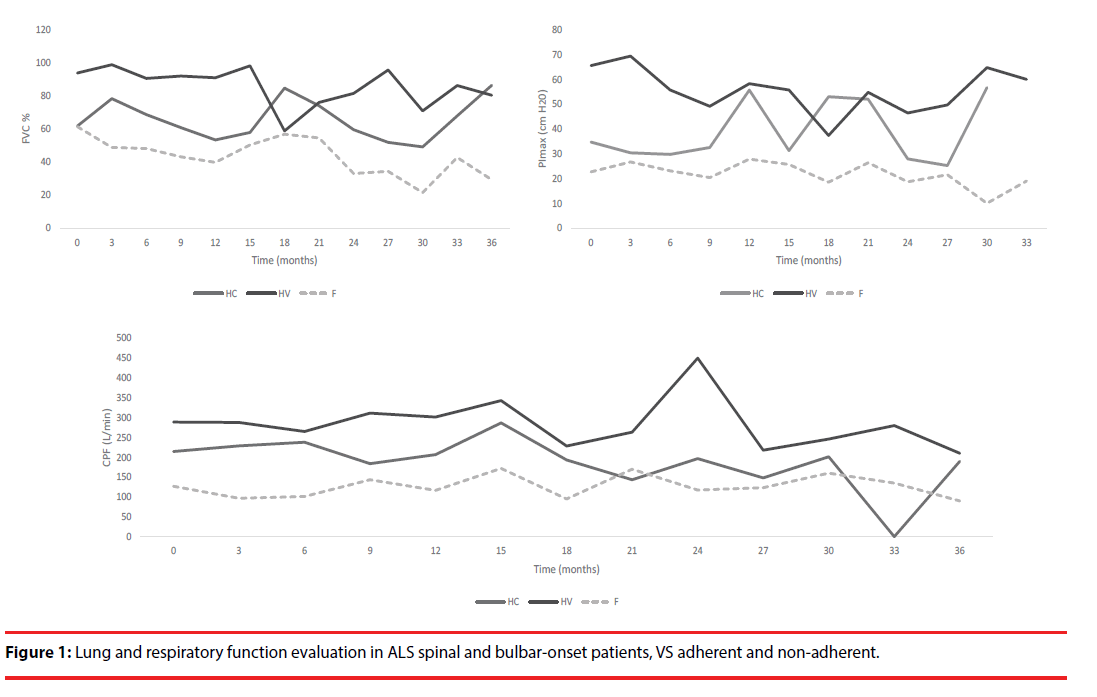
Regarding nocturnal hypoventilation group, VS was started even with normal FVC, leading to lung function maintenance or even improvement during the follow-up period. However, although a survival impact was observed, it was not statistically significant (Figures 1 and 2, Table 2).
| Parameters | HV | HC | F | p value |
|---|---|---|---|---|
| FVC (%) | 82.6 ± 17.7 | 66.7 ± 28.2 | 39.7 ± 14.0 | <0.001 |
| PImax (cm H2O) | 56.9 ± 25.2 | 34.7 ± 21.4 | 20.0 ± 16.4 | <0.001 |
| CPF (L/min) | 257.1 ± 64.5 | 225.6 ± 136.0 | 84.5 ± 91.5 | <0.001 |
| Time from diagnosis to VS (months) | 18.0 ± 20.0 | 6.3 ± 6.3 | 14.8 ± 15.3 | 0.147 |
| ABB: CPF: Cough Peak Flow; FVC: Forced Vital Capacity; PImax: Maximal Inspiratory Pressure; VS: Ventilatory Support; HV: Nocturnal Hypoventilation; HC: Diurnal Hypercapnia; F: Functional Compromise as Cause of VS | ||||
Table 2: Lung function parameters in the beginning of VS.
Figure 1: Lung and respiratory function evaluation in ALS spinal and bulbar-onset patients, VS adherent and non-adherent.
Considering the overall survival in ventilated patients, despite no statistically significant differences were found (p=0.212), those that had nocturnal hypoventilation criteria displayed a longer survival time (49.6 months) when compared to those evidencing functional compromise (34.8 months) or daytime hypercapnia (34.6 months) (Table 3). On the other hand, and looking at ALS phenotypes, bulbar-onset ALS patients showed a lower survival time when compared to spinal-onset patients (26.6 ± 22.7 vs. 44.6 ± 38.7 months, p=0.023) (Table 3).
| Parameters | Survival under VS (months) | p Value | Survival time (months) | p Value |
|---|---|---|---|---|
| Sex | 0.728 | 0.235 | ||
| Male | 27.2 ± 25.0 | 38.2 ± 36.3 | ||
| Female | 24.7 ± 22.5 | 29.6 ± 23.5 | ||
| Phenotype | 0.012 | 0.023 | ||
| Spinal-onset | 36.8 ± 25.8 | 44.6 ± 38.7 | ||
| Bulbar-onset | 19.4 ± 20.1 | 26.6 ± 22.7 | ||
| Factor to VS initiation | 0.338 | 0.339 | ||
| Hypercapnia | 30.7 ± 30.1 | 34.6 ± 30.2 | ||
| Hypoventilation | 31.6 ± 21.3 | 49.6 ± 33.4 | ||
| Functional | 21.0 ± 21.9 | 34.8 ± 31.4 | ||
| AHI residual | 0.040 | 0.238 | ||
| Yes | 17.7 ± 10.1 | 30.9 ± 22.0 | ||
| No | 29.2 ± 26.4 | 41.7 ± 34.3 | ||
| VS Adherent | 0.051 | 0.270 | ||
| Yes | 28.9 ± 26.6 | 40.4 ± 34.4 | ||
| No | 18.3 ± 10.0 | 31.2 ± 20.7 | ||
| VS adherence | 0.022 | 0.042 | ||
| Spinal-onset adherent | 42.4 ± 28.0 | 57.2 ± 40.9 | ||
| Spinal-onset non-adherent | 21.2 ± 6.4 | 29.2 ± 8.3 | ||
| Bulbar-onset adherent | 20.4 ± 22.2 | 29.7 ± 23.6 | ||
| Bulbar-onset non-adherent | 16.3 ± 12.1 | 32.7 ± 27.1 | ||
| ABB: AHI:Apnea-Hypopnea Index; VS: Ventilatory Support | ||||
Table 3: Survival-related factors.
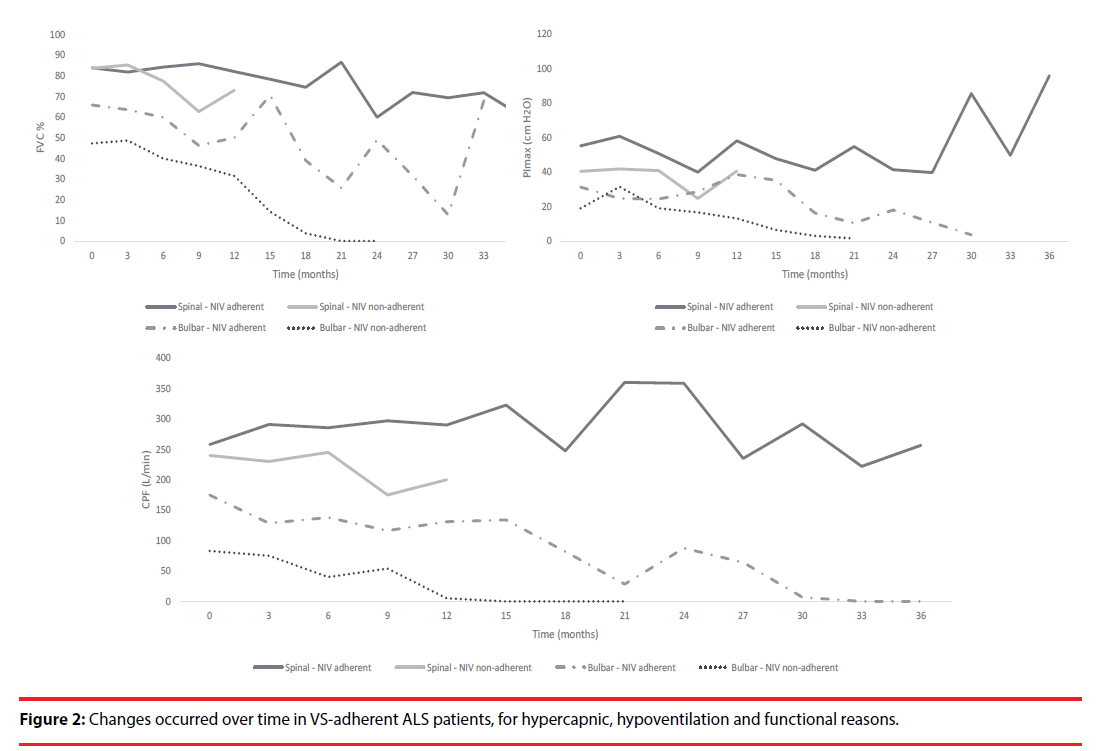
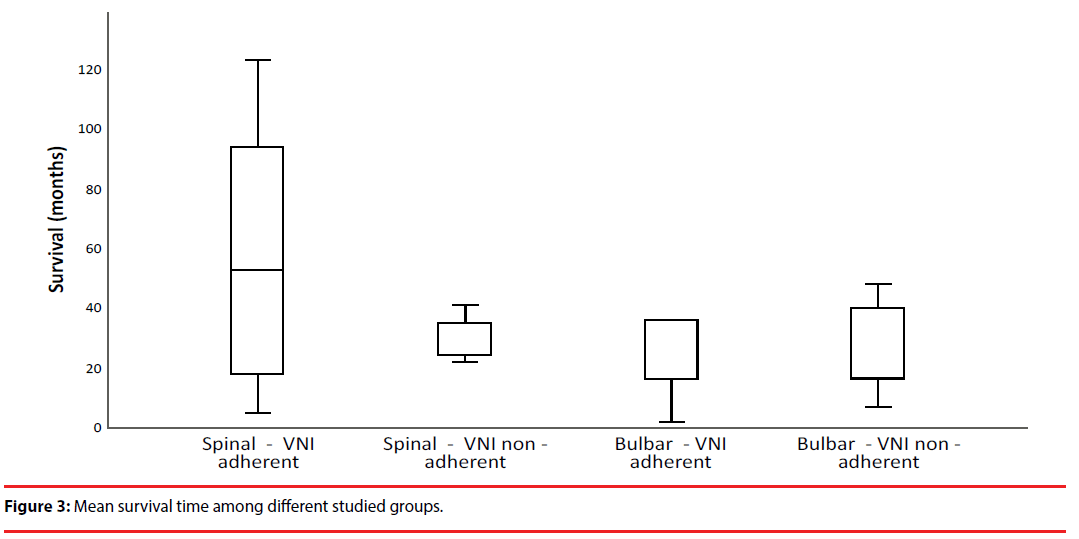
Indeed, functional impact and survival time were more evident in spinal-onset adherent than in non-adherent (57.2 ± 40.9 vs. 29.2 ± 8.3 months) or even in adherent bulbar-onset (29.7 ± 23.6 months) patients (p=0.042), meaning that VS adherence had a greater impact in overall survival (Table 3, Figure 3).
Given ALS progressive status, there was a fatal outcome in 48 patients (68.6%), mainly in bulbar-onset patients (n=30, 79.2%). The main causes of death were respiratory failure (n=40, 57.1%), followed by sepsis (n=3, 4.3%), sudden death (n=2, 2.9%), refractory heart failure (n=1, 1.4%), suicide (n=1, 1.4%), and intestinal occlusion (n=1, 1.4%). Only two patients were invasively ventilated, the others refused this option. All patients received palliative care, but only two were in a palliative care nursing home. Regarding admissions for respiratory exacerbations, during the follow-up period, from a total of 43 patients, 28 (65.1%) had a single episode, 13 (30.2%) had 2 and only 3 (7.0%) patients had 3 episodes. Those admissions were mostly found in bulbar-onset ALS patients (n=27, 55.1%), and 17 of them (34.7%) were previously ventilated due to functional criteria. In overall, spinal-onset ALS patients with effective VS use (VS adherent and without residual events) presented a better prognosis, with a global survival time of 57.2 ± 40.9 months (Table 3, Figure 3).
Discussion
In this study, males were dominant regarding the overall sample studied, and females in bulbar-onset ALS phenotype, which is in accordance with literature. Nevertheless, unlike other studies, bulbar-onset ALS was the most prevalent phenotype [1].
Patients were referred to the respiratory clinic on average 3 months after diagnosis, and the diagnosis was confirmed 13.7 months after the onset of symptoms; however, and according to the literature data, there was already a significant lung and muscle function impairment [1,29].
Regarding the overall survival time after VS initiation (25.8 ± 24.0 months), it was more evident in spinal-onset when compared to bulbar-onset (36.8 ± 26.8 vs. 19.4 ± 20.1 months) ALS patients. Similar findings were also reported by Sancho et al. [6], although greater benefits were observed in our study. According to the published evidence, spinal-onset ALS represents the phenotype with better outcomes and survival [1,2,5]. In ALS patients, VS has shown a great survival impact, even in bulbar-onset phenotype [6]. Furthermore, according to Georges et al. [30], the occurrence of residual events in ALS patients has been associated with a worse prognosis. In fact, VS is initiated based on international recommendations [8,13]; however, it appears that an early VS initiation, taking into account hypoventilation symptoms and nocturnal hypoventilation criteria (most commonly used in spinal-onset ALS patients), gives a more obvious functional and survival impact, although they have not been found statistical differences in this study, perhaps because of the small sample size. Interestingly, even in ventilated hypercapnic patients, VS initiation seems to contribute to lung function maintenance or even improvement, perhaps because FVC at the beginning of VS was only slightly decreased.
On the other hand, it has already been proven in previous studies that VS increases survival, using different pressures or volumes, and even distinct ventilatory modes [6,7,31]. In our study, VS outcomes were compared between three different groups of VS initiation criteria (i.e. functional, nocturnal hypoventilation and daytime hypercapnia), which is a strong point of this study, since, to the authors’ knowledge, it has never been assessed before. However, it has been demonstrated by Vitacca et al (2018) that an early VS initiation is beneficial in ALS patients, although only functional criteria (FVC<80% vs preserved function) were assessed by authors; nevertheless, survival impact was only stated in non-bulbar ALS patients [32].
Regarding the two different ALS phenotypes, bulbar-onset phenotype (which included pseudobulbar phenotype) was associated with worse prognosis, and VS efficacy with longer survival, which corroborates the literature data [5,6]. In fact, it has been reported, although differently, that VS promotes functional and arterial blood gas improvement/stability, mainly in spinal-onset adherent patients, as previously described [6,33–35].
However, contrary to expectations, non-adherent bulbar-onset patients had a longer survival than adherent bulbar-onset patients, which may be just related to the sample size (only 4 non-adherent and 22 adherent bulbar-onset patients). Therefore, further evaluations are needed.
As main study limitations, we recognize the small sample size and the fact that it is a single center cohort, which may compromise data robustness and predictability. Thus, by enlarging the sample size and expanding to other centers, it may be possible to improve the statistical power and to achieve the odds ratio related to better survival.
Conclusions
Although ALS is a progressive neurodegenerative and fatal disease, we found a prominent functional improvement after VS start in a significant fraction of our cohort, especially in spinalonset adherent patients and without residual events. In addition, as nocturnal hypoventilation symptoms determine the need for VS initiation, and as we found functional benefits, its future use should be clearly highlighted towards an effective early intervention. Lung and muscle function impairment represents a late VS criterion, and may have an impact in overall ALS prognosis, suggesting the screen of nocturnal hypoventilation in ALS patients with sleep studies and capnography. Anyway, non-invasive VS seems to be effective, even in bulbar-onset ALS patients, 24 h ventilated, clearly improving the prognosis of this fatal neurodegenerative disease. Further studies are needed to deepen knowledge on bulbar-onset ALS patients, in whom non-invasive VS is ineffective, and to determine the cause for this ineffectiveness.
Acknowledgments
Martins N. would like to thank the Portuguese Foundation for Science and Technology (FCT–Portugal) for the Strategic project ref. UID/BIM/04293/2013 and “NORTE2020— Programa Operacional Regional do Norte” (NORTE-01-0145-FEDER-000012).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N. Engl. J. Med 377(2), 162-172 (2017).
- Gordon PH. Amyotrophic lateral sclerosis : an update for 2013 clinical features , pathophysiology , management and therapeutic trials. Aging. Dis 4(5), 295-310 (2013).
- Review AS. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis. Arch. Neurol 69(11), 1410–1416 (2012).
- Geevasinga N, Loy CT, Menon P, et al. Clinical neurophysiology awaji criteria improves the diagnostic sensitivity in amyotrophic lateral sclerosis : A systematic review using individual patient data. Clin. Neurophysiol 127(7), 2684-2691 (2016).
- Ravits J, Appel S, Baloh RH, et al. Deciphering amyotrophic lateral sclerosis : What phenotype , neuropathology and genetics are telling us about pathogenesis. Amyotroph. Lateral. Scler. Frontotemporal. Degener 14(1), 5-18 (2013).
- Sancho J, Martínez D, Bures E, et al. Bulbar impairment score and survival of stable amyotrophic lateral sclerosis patients after noninvasive ventilation initiation. Eur. Resp. Society 4(2), 159 (2018).
- Zoccolella S, Beghi E, Palagano G, et al. Analysis of survival and prognostic factors in amyotrophic lateral sclerosis : a population based study. J. Neurol. Neurosurg. Psychiatry 79(1), 33-37 (2008).
- Jackson CE, Kasarskis EJ. Practice Parameter update : The care of the patient with amyotrophic lateral sclerosis : Drug , nutritional , and respiratory therapies ( an evidence-based review ) Report of the Quality Standards Subcommittee of the American Academy of Neurology. (2009).
- Orlikowski D, Prigent H, Salva MQ, et al. Prognostic value of nocturnal hypoventilation in neuromuscular patients. Neuromuscul. Disord 27(4), 326-330 (2017).
- Ward S, Chatwin M, Heather S, et al. Randomised controlled trial of non-invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime normocapnia. Thorax 60(12), 1019-1024 (2005).
- Nardi J, Prigent H, Adala A, et al. Nocturnal oximetry and transcutaneous carbon dioxide in home-ventilated neuromuscular patients. Respir. Care 57(9), 1425-1430 (2012).
- Simonds AK. Chronic hypoventilation and its management. Eur. Respir. Rev 22(129), 325-332 (2013).
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 aasm manual for the scoring of sleep and associated events deliberations of the sleep apnea defi nitions task force of the american academy of sleep medicine. J. Clin. Sleep Med 8(5), 597-619 (2012).
- Calvo A, Moglia C, Lunetta C, et al. Factors predicting survival in ALS : a multicenter Italian study. J. Neurol 264(1), 54-63 (2017).
- Polkey MI, Lyall RA, Yang K, et al. Respiratory muscle strength as a predictive biomarker for survival in amyotrophic lateral sclerosis. Am. J. Respir. Crit. Care Med 195(1), 86-95 (2017).
- Traynor BJ, Corr B. An outcome study of riluzole in amyotrophic lateral sclerosis. J. Neurol 250(4), 473-479 (2003).
- Chen L, Liu X, Tang L, et al. Long-term use of riluzole could improve the prognosis of sporadic amyotrophic lateral sclerosis patients : a real-world cohort study in China. Front. Aging. Neurosci 8(1), 246 (2016).
- Hinchcliffe M, Smith A. Riluzole : real-world evidence supports significant extension of median survival times in patients with amyotrophic lateral sclerosis. Degener. Neurol. Neuromuscul. Dis 7(1), 61–70 (2017).
- Groenestijn AC Van, Port IGL Van De, Schröder CD, et al. Effects of aerobic exercise therapy and cognitive behavioural therapy on functioning and quality of life in amyotrophic lateral sclerosis : protocol of the FACTS-2-ALS trial. BMC. Neurol 11(1), 70 (2011).
- Farrero E, Prats E, Povedano M, et al. Survival in amyotrophic lateral sclerosis with home mechanical ventilation: the impact of systematic respiratory assessment and bulbar involvement. Chest 127(1), 2132-2138 (2005).
- Winck JC, LeBlanc C, Soto JL, et al. The value of cough peak flow measurements in the assessment of extubation or decannulation readiness. Rev. Port. Pneumol 21(2), 94-98 (2015).
- Suarez A, Monteiro SG. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am. J. Phys. Med. Rehabil 81(7), 506-511 (2002).
- Morgan RK, McNally S, Alexander M, et al. Use of sniff nasal-inspiratory force to predict survival in amyotrophic lateral sclerosis. Am. J. Respir. Crit. Care Med 171(3), 269-274 (2005).
- Varrato J, Siderowf A, Damiano P, et al. Postural change of forced vital capacity predicts some respiratory symptoms in ALS. Neurology 57(2), 357-359 (2001).
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur. Respir. J 26(2), 319-338 (2005).
- Gibson GJ, Whitelaw W, Siafakas N, et al. American Thoracic Society / European Respiratory Society ATS / ERS Statement on Respiratory Muscle Testing. Am. J. Respir. Crit. Care Med 166(1), 518-624 (2002).
- Finder J, Gozal D, Iber C, et al. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation ( NPPV ) in stable chronic alveolar hypoventilation syndromes. 6(5) (2010).
- Aboussouan LS. Sleep-disordered breathing in neuromuscular disease. Am. J. Respir. Crit. Care Med 191(9), 979-989 (2015).
- Fiorenza D, Vitacca M, Bianchi L, et al. Lung function and disability in neuromuscular patients at first admission to a respiratory clinic. Respir. Med 105(1), 151-158 (2011).
- Georges M, Attali V, Golmard JL, et al. Reduced survival in patients with ALS with upper airway obstructive events on non-invasive ventilation. J. Neurol. Neurosurg. Psychiatry 87(10), 1045-1050 (2016).
- Sancho J, Servera E, Morelot-panzini C, et al. Non-invasive ventilation effectiveness and the effect of ventilatory mode on survival in ALS patients. Amyotroph. Lateral. Scler. Frontotemporal. Degener 15(1-2) 55-61 (2014).
- Vitacca M, Alessandra M, Lunetta C, et al. Impact of an early respiratory care program with NIV adaptation in patients with ALS. Eur. J. Neurol 25(3), 556-533 (2018).
- Vitacca M, Clini E, Facchetti D, et al. Breathing pattern and respiratory mechanics in patients with amyotrophic lateral sclerosis. Eur. Respir. J 10(7), 1614-1621 (1997).
- Vitacca M, Vianello A. Respiratory outcomes of patients with amyotrophic lateral sclerosis: an italian nationwide survey. Respir. Care 58(9), 1433-1441 (2013).
- Gruis KL, Brown DL, Schoennemann A, et al. Predictors of noninvasive ventilation tolerance in patients with amyotrophic lateral sclerosis. Muscle and Nerve 32(6), 808-811 (2005).