Review Article - Interventional Cardiology (2024) Volume 16, Issue 3
Ventricular pre-excitation in asymptomatic pediatric patients and its current management
- Corresponding Author:
- Sanam Safi
Department of Pediatrics and Adolescent Medicine, Section of Pediatric Cardiology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark,
E-mail: sanam.safi@regionh.dk
Received date: 15-May-2024, Manuscript No. FMIC-24-135227; Editor assigned: 17-May-2024, PreQC No. FMIC-24-135227 (PQ); Reviewed date: 31-May-2024, QC No. FMIC-24-135227; Revised date: 07-Jun-2024, Manuscript No. FMIC-24-135227 (R); Published date: 14-Jun-2024, DOI: 10.37532/1755-5310.2023. 16(3).745
Abstract
The management of asymptomatic children with Ventricular Pre-Excitation (VPE) as well as prophylactic ablation amongst these patients has previously been less well established. Mandatory risk stratification for sudden cardiac death is now a key component of care, and due to the limitations of non-invasive methods, invasive Electrophysiology Study (EPS) has become the preferred diagnostic approach. However, since EPS lacks a 100% sensitivity and the risks associated with ablation are generally low, proceeding with catheter ablation following an invasive EPS should be considered. The decision to perform ablation should consider several factors such as the location of the Accessory Pathways (APs), the number of APs, AP conduction properties, co-existing structural heart disease, the patient’s weight/age, and family preferences. These factors must be balanced against the potential risks associated with procedural complications and the future risk of arrhythmias. The aim of this review article is to discuss the current recommendations and management of asymptomatic children with VPE and to point out the lack of evidence and the need for future research within this group of patients
Keywords
Ventricular pre-excitation • Pediatric • Electrophysiology
Introduction
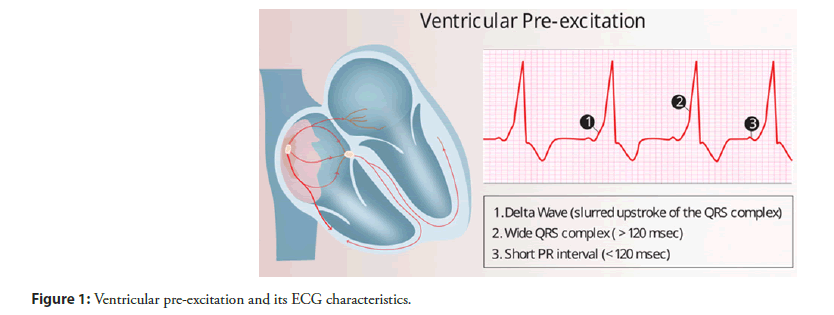
Ventricular Pre-Excitation (VPE), a premature activation of the ventricles, indicates the presence of one or more Accessory Pathways (AP) connecting the atria and ventricles outside of the Atrioventricular (AV) node [1]. This condition predisposes individuals to paroxysmal Supraventricular Tachycardia (SVT), and when accompanied by symptoms of SVT such as palpitations, chest discomfort, and dizziness, it is defined as Wolff-Parkinson-White (WPW) syndrome [2,3]. VPE is characterized on a 12-lead ECG by a delta wave, short PR interval, and widened QRS complex, (Figure 1) [1].
The prevalence of VPE is estimated at 0.1% to 0.3% of the general population [4-7]. A significant proportion of individuals diagnosed with VPE are asymptomatic and often incidentally found during routine ECG examination. More specifically, up to 65% ≤ 18 years and 40% of adults ≥ 30 years are found to have asymptomatic VPE patterns on their ECGs [8-10]. WPW syndrome is more common among males, children, and young adults, as well as in patients with congenital heart defects such as Ebstein’s anomaly and hypoplastic left heart syndrome [1,7,9,11]. Atrioventricular Re-Entrant Tachycardia (AVRT) is the predominant arrhythmia in WPW syndrome, occurring in approximately 80% to 95% of casesn [1,12,13]. This is closely followed by Atrial Fibrillation (AF), which, although extremely rare in the general pediatric population, has been found to have incidence rates ranging from 1.5% to 12% in children with WPW [10,14,15]. In adults with WPW, the incidence of AF can increase to as high as 30%, highlighting the escalating risk of AF with age among these patients [9,12,16- 20]. Patients with VPE are particularly susceptible to complications from AF as it can progress to pre-excited AF and degenerate further into Ventricular Fibrillation (VF), leading to cardiac arrest and Sudden Cardiac Death (SCD) [21,22]. APs can be removed by ablation treatment, thus eliminating the risk of pre-excited AF and SCD [23,24]. However, the risk of SCD in children with VPE is low, and the potential ablation treatment is an invasive procedure with risks of procedure-related complications [25]. Hence, the purpose of this review article is to discuss the current recommendations and management of asymptomatic children with VPE and to point out the lack of evidence and the need for future research within this group of patients. This paper was an invited paper based on our previously published article in the Ugeskrift for Laeger, a Danish medical journal [26].
Pathophysiology
In a healthy heart, the AV node is the only electrical connection between the atria and ventricles. In VPE, one or more APs typically consisting of myocardial cells persist after birth [27]. These pathways are most commonly located on the left free wall of the heart (58%), but can also be found posteroseptally (24%), on the right free wall (13%), and anteroseptally (8%) [28,29]. The conductivity of these pathways varies significantly and is an important factor when assessing the risk of SCD in these patients [24]. The location of the AP alone is insufficient to determine the risk for SCD. However, ECG characteristics that indicate the presence of a fasciculoventricular AP, for instance, have not been linked to an increased risk for SCD or tachyarrhythmias, and therefore, might not necessitate treatment [30,31]. APs can cause premature activation of the ventricles, circumventing the typical AV node delay and leading to a slower propagation of impulses as the rapid transmission through the his bundle and bilateral conduction pathways is partially bypassed. VPE manifests through these pathways in various forms: It may be present consistently during sinus rhythm, occur intermittently, or involve only retrograde electrical conduction. The latter known as “concealed VPE,” yields a normal resting-ECG as it does not impact antegrade electrical impulse conduction [32]. APs can facilitate re-entrant tachycardia via macro-reentrant circuits using either an antegrade or retrograde impulse direction over AP, resulting in AVRT. Orthodromic AVRT, where impulses traverse retrograde through the AP, produces a narrow complex tachycardia and accounts for about 90% of cases. The remaining 10%, known as antidromic AVRT, involves antegrade conduction through the AP, creating a wide complex tachycardia (Figure 2). No likely symptomatic difference is seen between orthodromic and antidromic AVRT, although the former resembles classical SVT, while the latter may mimic ventricular tachycardia [32-34].
Risk of sudden cardiac death in asymptomatic children with ventricular pre-excitation
Children with VPE who develop AF, face a risk of rapid, antegrade conduction across the AP to the ventricles which, due to the lack of delay in the AV node, can degenerate into VF and SCD [35,36]. Studies have shown that children with VPE have a significantly higher risk of SCD compared to those without VPE [14,37]. However, determining the absolute risk is challenging without extensive screening programs due to difficulties in accurately identifying asymptomatic cases. Adults with WPW syndrome are estimated to have a lifetime risk of SCD of 3% to 4% (0.25% per patient-year) [38]. Although asymptomatic individuals with VPE are generally deemed to be at a lower risk, with SCD incidence reported between 0.0012% and 0.6% annually, it is noteworthy that SCD may still be present as the initial symptom in these cases [37,39,40]. Furthermore, a meta-analysis demonstrated that asymptomatic children with VPE face an increased risk of SCD compared to asymptomatic adults with VPE, with incidence rates reported at 1.93 and 0.86, respectively [38]. Children with asymptomatic VPE and a structurally normal heart have an overall incidence risk of SCD of 1.1 per 1000 patient-years [15], while children with asymptomatic VPE and associated structural heart disease face a significantly increased rate of 27 per 1000 patientyears. These findings challenge previous perceptions and highlight a discrepancy in risk assessments between different age groups and while older guidelines considered children ≤ 12 years old at negligible risk of SCD, recent publications indicate that the highest risk profile might be in younger children [24,41].
Risk stratification for sudden cardiac death in asymptomatic children with ventricular pre-excitation
To identify children at increased risk of life-threatening arrhythmias and SCD, risk stratification of asymptomatic children with VPE is recommended [13,42-44]. Risk stratification can be performed both non-invasively and invasively and is recommended in children earliest from the age of 8-10 years [3,43,44]. The general trend is towards the use of invasive risk stratification, which is now recommended with a class IIa Level of Evidence (LOE) B recommendation for asymptomatic patients with VPE and a class I LOE B recommendation for patients at high risk (e.g., athletes, pilots, professional drivers) [12,45]. Noninvasive tests including 12-lead ECG to localize APs, Holter monitoring to detect intermittent loss of VPE, and exercise testing to detect sudden loss of the delta wave at high heart rates, may be considered as a part of the initial phase of risk stratification recommended with a class IIb LOE B recommendation [12]. Loss of the delta wave during stress tests indicates a long refractory period/slow conduction through AP and thus a poorer/absent ability for rapid, antegrade conduction over AP and a lower risk of rapid ventricular arrhythmia and SCD with AF. Gradual loss of the delta wave may, however, potentially reflect faster AV node conduction with increased sympathetic stimulation and thus mask preexcitation [1]. The effective refractory period of APs (APERP) can be indirectly assessed as ≥ 250 ms and thereby of low risk with complete loss of the delta wave during an exercise test.
Persistence of VPE during stress tests has been found to have a sensitivity of 96% and Negative Predictive Value (NPV) of 88% but a specificity and Positive Predictive Value (PPV) of only 17% and 40%, respectively, when identifying patients at high risk of SCD [46]. However, when comparing noninvasive testing with invasive Electrophysiological Study (EPS) for identifying patients at high risk, similar reported limitations were found in 2019 by LaRocca and colleagues who found a low specificity of 27% and PPV of 18%, while the sensitivity and NPV continued to be high at 80% and 87%, respectively [47].
Intermittent VPE observed on Holter monitoring or loss of VPE during exercise testing has previously been considered a low-risk AP, suggesting no further evaluation [3,48]. However, recent studies indicate that the prevalence of high-risk APs (APERP ≤ 250 ms) in patients with intermittent VPE is comparable to that in patients with persistent VPE [49-52]. Hence, the presence of intermittent VPE does not alleviate the risk of SCD in these patients.
During invasive EPS, the APERP and the Shortest Pre-Excited R-R Interval (SPERRI) can be assessed during spontaneous AF or with adrenergic stimulation using isoproterenol [3,31]. However, the latter is rarely employed in practice, and its role in risk stratification for asymptomatic patients is yet to be clearly defined [22,31,53]. In addition, the number and location of APs and their conduction properties during sinus rhythm/atrial pacing are determined. A high-risk AP is characterized by several characteristics including the presence of multiple APs, septal location, the provocation of tachyarrhythmias, retrograde AP conduction, SPERRI-AF and/ or APERP ≤ 250 ms or ≤ 220 ms during adrenergic stimulation [3,31]. However, studies have found that SPERRI ≤ 220 ms-250 ms during AF is more effective than APERP when considering high-risk AP with sensitivities as high as 100% in children with WPW syndrome, although lower in asymptomatic patients with VPE [36,54-57]. Despite lacking 100% sensitivity in identifying asymptomatic children with VPE at risk of SCD, invasive EPS is considered the standard for risk stratification and has become the preferred diagnostic approach among electrophysiologists [45]. The purpose is to evaluate the risk of SCD induced by pre-excited AF with the possibility of ablation in the same procedure. A survey among pediatric electrophysiologists from 2018 showed that 22% would choose to ablate all APs irrespective of EPS findings, while 61% indicated they would always attempt to ablate a right-sided AP regardless of EPS findings, 58% would consistently ablate a left-sided AP, but only 23% would choose to ablate a septal AP [45]. The remaining electrophysiologists would only ablate an AP depending on the measured SPERRI and APERP with a cut-off of 250 ms for all measures at all locations, while SPERRI-AF ≤ 250 ms or APERP ≤ 250 ms alone as a sufficient measure to warrant ablation was considered by 86% and 76%, respectively [45].
Treatment of asymptomatic children with ventricular preexcitation
In the 1990s, percutaneous Radiofrequency Ablation (RFA) via catheterization through the femoral vein was introduced as a curative treatment for arrhythmias in children with WPW syndrome [24]. Subsequently, similar treatment became possible with cryotherapy [31]. Since then, it has been widely accepted as the primary treatment for children with WPW syndrome due to its high success rates and low risk profile, thus eliminating the earlier need for surgical ablation and pharmacological antiarrhythmic treatment [23,31,58]. Registries such as the Multicenter Pediatric and Congenital EP Quality Initiative from 2016 have documented an acute success rate of ≥ 95% (follow-up success rate of 93%) for RFA of AP in patients with VPE, achieving the highest rates of 97% for procedures performed on left-sided APs with complications arising in 2.5% of cases [58]. Minor complications associated with ablation typically include bleeding at the access site and local infection, while the more serious complications necessitating emergency or ongoing treatment include AV block (second and/or third degree), catheter perforation or pericardial effusion, and thromboembolic events [24,58,59]. Notably, an improvement in safety is evident, with complication rates decreasing from 4% during 1991-1995 to 3.2% in 1996-1999, reflecting advances in technology and increased procedural experience [59]. In Denmark, between 35 and 60 children with WPW syndrome undergo ablation treatment each year. Over the past five years, no severe complications have been reported from these procedures. Additionally, advancements in technique have reduced the need for fluoroscopic exposure during ablations, with current procedures typically requiring less than 5 minutes, and often under 2 minutes, per case [58]. More and more centers now perform zero-fluoroscopic ablation strategies in children.
Guidelines and evidence supporting prophylactic ablation for children with VPE are not uniformly clear or definitive [3,12,22,31,60]. Ablation can eliminate the risk of severe arrhythmias and SCD and provide reassurance to patients and their families. Furthermore, a study assessing quality of life in children with VPE found a significant disparity in their general health satisfaction compared to healthy controls. Additionally, these patients exhibited higher levels of sadness and nervousness than their healthy peers [61]. However, since the risk of lifethreatening arrhythmias in asymptomatic children with VPE is low, and ablation is an invasive procedure with risks of complications, a more conservative approach involving careful monitoring of asymptomatic patients without early intervention has previously been argued for [62,63]. In a Randomized Controlled Trial (RCT) exploring the effects of prophylactic ablation versus no ablation in individuals aged 14 to 34 years with asymptomatic VPE and inducible arrhythmia, a risk reduction of 92% was observed among those who underwent ablation (p<0.001), with a five-year arrhythmic incidence including AVRT, SVT, AF, and VF of 7% among the treated and 77% in the control group [64]. Another RCT assessing prophylactic catheter ablation in children with asymptomatic VPE reported a similar higher arrhythmic event rate of 44% in the control group during a median follow-up of 19 months compared to 5% during a 34-month follow-up in the ablation group [40]. Additionally, two patients out of the 27 patients in the control group experienced VF and one patient suffered SCD [40].
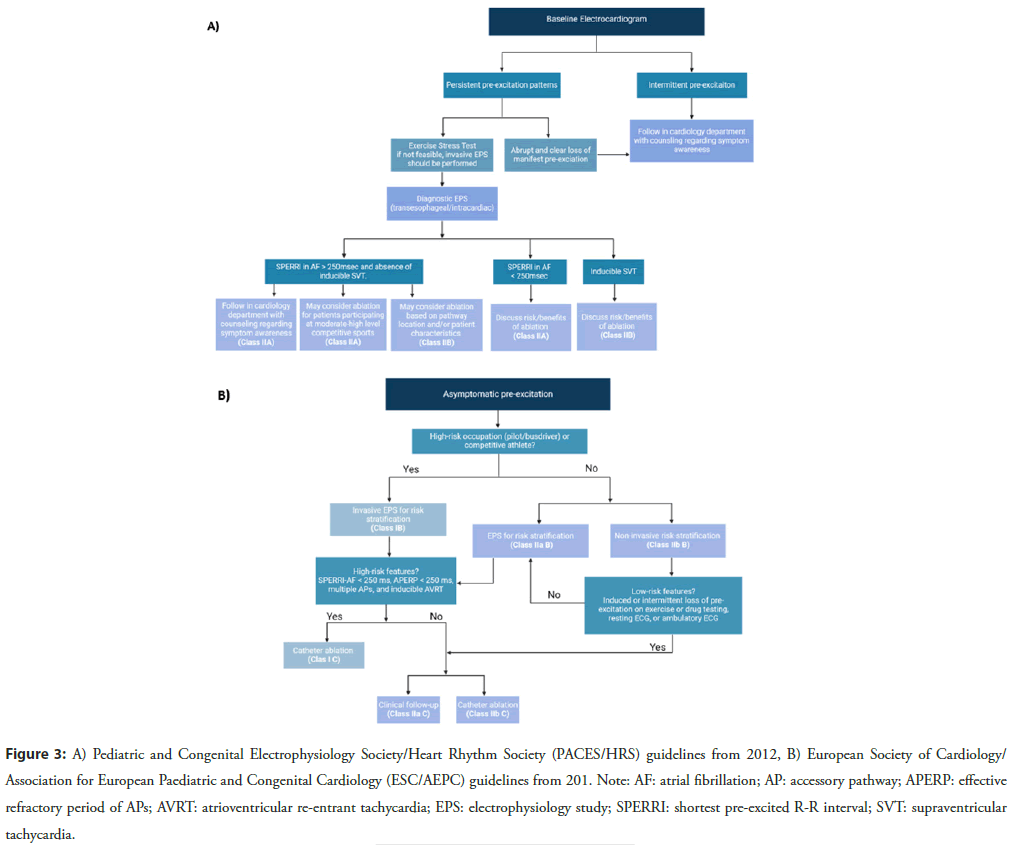
Over the last two decades, recommendations for prophylactic ablation in asymptomatic pediatric patients with VPE have evolved substantially. The 2019 guidelines from the European Society of Cardiology/Association for European Paediatric and Congenital Cardiology (ESC/AEPC), applicable to both children and adults, recommends prophylactic ablation of APs with highrisk characteristics such as SPERRI/APERP ≤ 250 ms, multiple APs, and inducible AP-mediated tachycardia in asymptomatic children, with a Class I LOE B and for asymptomatic VPE patients in high-risk occupations (e.g., elite athletes or professional drivers) with a Class I LOE C [12]. These recommendations were absent in the earlier guidelines from the Pediatric and Congenital Electrophysiology Society/Heart Rhythm Society (PACES/HRS) of 2012 and 2016, which focused on children and adolescents aged 8 to 21 years [3,31]. In asymptomatic children and adults with VPE and with low-risk AP characteristics or if there is a strong patient/family preference, catheter ablation may be considered with a Class IIb LOE C recommendation according to the most recent ESC/AEPC guidelines [12]. The PACES/HRS guidelines from 2012 and 2016 suggest that ablation may be considered in asymptomatic VPE children with SPERRI ≥ 250 ms with a Class IIb LOE C/E, while those with SPERRI ≤ 250 ms during AF are advised for ablation with a Class IIa LOE B/C (Figure 3) [3,31].
Figure 3: A) Pediatric and Congenital Electrophysiology Society/Heart Rhythm Society (PACES/HRS) guidelines from 2012, B) European Society of Cardiology/ Association for European Paediatric and Congenital Cardiology (ESC/AEPC) guidelines from 201. Note: AF: atrial fibrillation; AP: accessory pathway; APERP: effective refractory period of APs; AVRT: atrioventricular re-entrant tachycardia; EPS: electrophysiology study; SPERRI: shortest pre-excited R-R interval; SVT: supraventricular tachycardia.
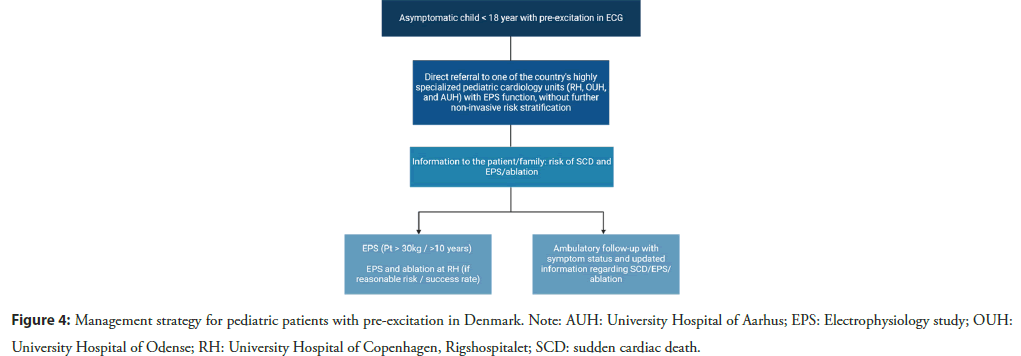
The PACES/HRS committee considered age a critical factor when recommending ablation in the 2012 guidelines, whereas the revision from 2016 suggested that patient weight is a more significant determinant of the risk-benefit ratio for ablation, recommending procedures in patients from approximately 15 kg. In Denmark, EPS/ablation is typically performed on children weighing ≥ 30 kg or who are ≥ 10 years of age due to the increased risk of complications in smaller children. A more detailed management strategy for pediatric patients with pre-excitation in Denmark, where all ablation on pediatric patients has been centralized to the University Hospital of Copenhagen, Rigshospitalet, is depicted in Figure 4.
Conclusion
The management of asymptomatic children with VPE as well as prophylactic ablation amongst these patients has previously been less well established. Mandatory risk stratification for SCD is a key component of care, and due to the limitations of non-invasive methods, invasive EPS has become the preferred diagnostic approach. However, since EPS lacks 100% sensitivity and the risks associated with ablation are minimal, proceeding with catheter ablation following an invasive EPS should be considered. The decision to perform ablation should consider several factors such as the location of APs, the number of APs, AP conduction properties, co-existing structural heart disease, the patient’s weight/age, and family preferences. These factors must be balanced against the potential risks of procedural complications and the future risk of arrhythmias. In case of doubt before referral to EPS, consultation with electrophysiologists and pediatric cardiologists at a highly specialized unit is recommended. Further research, better risk stratification, and the development of better and safer ablation tools, such as pulse field ablation, are needed to improve the balance between benefits and risks associated with prophylactic ablation in asymptomatic children with VPE. This is significant in order to refine and clarify guidelines, ensuring the optimization of timing for risk stratification and treatment within this patient group.
References
- Yadav V, Thapa S, Gajurel RM, et al. A Wolff-Parkinson-White (WPW) electrocardiographic pattern in asymptomatic patient-state-of-the-art-review. Cardiol Cardiovasc Med.7(2):046-053 (2022).
- Al-Khatib SM, Pritchett EL. Clinical features of wolff-parkinson-white syndrome. Am Heart J.138(3):403-413 (1999).
- Cohen MI, Triedman JK, Cannon BC, et al. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: Developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society. Heart rhythm.9(6):1006-1024 (2012).
- Kobza R, Toggweiler S, Dillier R, et al. Prevalence of pre-excitation in a young population of male Swiss conscripts. Pacing Clin Electrophysiol.34(8):949-953 (2011).
- Davenport ED, Rupp KA, Palileo E, et al. Asymptomatic Wolff-Parkinson-white pattern ECG in USAF aviators. Aerosp Med Hum Perform.88(1):56-60 (2017).
- Averill KH, Fosmoe RJ, Lamb LE, et al. Electrocardiographic findings in 67,375 asymptomatic subjects: IV. Wolff-Parkinson-White syndrome. Am J Cardiol.6(1):108-129 (1960).
- Skov MW, Rasmussen PV, Ghouse J, et al. Electrocardiographic pre-excitation and risk of cardiovascular morbidity and mortality: Results from the Copenhagen ECG Study. Circ Arrhythm Electrophysiol.10(6):e004778 (2017).
- Munger TM, Packer DL, Hammill SC. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota,1953-1989. Circulation.87:866-873 (1993).
- Goudevenos JA, Katsouras CS, Graekas G, et al. Ventricular pre-excitation in the general population: A study on the mode of presentation and clinical course. Heart.83(1):29-34 (2000).
- Santinelli V, Radinovic A, Manguso F, et al. The natural history of asymptomatic ventricular pre-excitation. A long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol.53:275-280 (2009).
- Novella J, DeBiasi RM, Coplan NL, et al. Non-invasive risk stratification for sudden death in asymptomatic patients with Wolff-Parkinson-White syndrome. Rev Cardiovasc Med.15(4):283-289 (2014).
- Brugada J, Katritsis DG, Arbelo E, et al. 2019 ESC guidelines for the management of patients with supraventricular tachycardia the task force for the management of patients with supraventricular tachycardia of the European society of Cardiology (ESC) developed in collaboration with the association for European paediatric and congenital Cardiology (AEPC). Eur Heart J.41(5):655-720 (2020).
- Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias-executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation.108(15):1871-909 (2003).
- Janson CM, Millenson ME, Okunowo O, et al. Incidence of life-threatening events in children with Wolff-Parkinson-White syndrome: Analysis of a large claims database. Heart Rhythm.19(4):642-647 (2022).
- Cain N, Irving C, Webber S, et al. Natural history of Wolff-Parkinson-White syndrome diagnosed in childhood. Am J Cardiol.112(7):961-965 (2013).
- Sharma AD, Klein GJ, Guiraudon GM, et al. Atrial fibrillation in patients with Wolff-Parkinson-White syndrome: Incidence after surgical ablation of the accessory pathway. Circulation.72(1):161-169 (1985).
- Campbell RW, Smith RA, Gallagher JJ, et al. Atrial fibrillation in the pre-excitation syndrome. Am J Cardiol.40(4):514-520 (1977).
- Bauernfeind RA, Wyndham CR, Swiryn SP, et al. Paroxysmal atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol.47(3):562-569 (1981).
- Robinson KI, Rowland ED, Krikler DM, et al. Wolff-Parkinson-White syndrome: Atrial fibrillation as the presenting arrhythmia. Heart.59(5):578-580 (1988).
- Della Bella P, Brugada P, Talajic M, et al. Atrial fibrillation in patients with an accessory pathway: Importance of the conduction properties of the accessory pathway. J Am Coll Cardiol.17(6):1352-1356 (1991).
- Delise P, Sciarra L. Sudden cardiac death in patients with ventricular pre-excitation. Card Electrophysiol Clin.12(4):519-525 (2020).
- Brugada J, Keegan R. Asymptomatic ventricular pre-excitation: Between sudden cardiac death and catheter ablation. Arrhythm Electrophysiol Rev.7(1):32 (2018).
- Telishevska M, Hebe J, Paul T, et al. Catheter ablation in ASymptomatic PEDiatric patients with ventricular preexcitation: Results from the multicenter “CASPED” study. Clin Res Cardiol.108:683-690 (2019).
- Pappone C, Vicedomini G, Manguso F, et al. Wolff-Parkinson-White syndrome in the era of catheter ablation: Insights from a registry study of 2169 patients. Circulation.130(10):811-819 (2014).
- Rodriguez-Gonzalez M, Castellano-Martinez A, Perez-Reviriego AA, et al. Risk-stratification strategy for sudden cardiac death in the very young children with asymptomatic ventricular preexcitation. Curr Cardiol Rev.16(2):83-89 (2020).
- Safi S, Jøns C, Jacobsen PK, et al. Asymptomatic children with ventricular pre-excitation/WPW pattern. Ugeskr Laeger.186(3):V08230550 (2024).
- Mirzoyev S, McLeod CJ, Asirvatham SJ, et al. Embryology of the conduction system for the electrophysiologist. Indian Pacing Electrophysiol J.10(8):329 (2010).
- Cain ME, Luke RA, Lindsay BD, et al. Diagnosis and localization of accessory pathways. Pacing Clin Electrophysiol.15(5):801-824 (1992).
- de Chillou C, Rodriguez LM, Schläpfer J, et al. Clinical characteristics and electrophysiologic properties of atrioventricular accessory pathways: Importance of the accessory pathway location. J Am Coll Cardiol.20(3):666-671 (1992).
- O’Leary ET, Dewitt ES, Mah DY, et al. Differentiation of fasciculoventricular fibers from anteroseptal accessory pathways using the surface electrocardiogram. Heart Rhythm.16(7):1072-1079 (2019).
- Saul JP, Kanter RJ, Abrams D, et al. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease. Heart Rhythm.13(6):e251-e289 (2016).
- Chugh A, Morady F. Preexcitation, atrioventricular reentry, and variants.(2014).
- Jabbour F, Grossman SA. Atrioventricular reciprocating tachycardia. (2024).
- Bardy GH, Packer DL, German LD, et al. Preexcited reciprocating tachycardia in patients with Wolff-Parkinson-White syndrome: Incidence and mechanisms. Circulation.70(3):377-391 (1984).
- Dreifus LS, Haiat R, Watanabe Y, et al. Ventricular fibrillation: A possible mechanism of sudden death in patients with Wolff-Parkinson-White syndrome. Circulation.43(4):520-527 (1971).
- Klein GJ, Bashore TM, Sellers TD. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med.301(20):1080-1085 (1979).
- Etheridge SP, Escudero CA, Blaufox AD, et al. Life-threatening event risk in children with Wolff-Parkinson-White syndrome: A multicenter international study. JACC Clin Electrophysiol.4(4):433-444 (2018).
- Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: A meta-analysis. Circulation.125(19):2308-2315 (2012).
- Delise P, Sciarra L. Asymptomatic Wolff-Parkinson-White: what to do. Extensive ablation or not? J Cardiovasc Med (Hagerstown). 8(9):668-674 (2007).
- Pappone C, Manguso F, Santinelli R, et al. Radiofrequency ablation in children with asymptomatic Wolff-Parkinson-White syndrome. N Engl J Med.351(12):1197-1205 (2004).
- Pappone C, Santinelli V, Rosanio S, et al. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: Results from a large prospective long-term follow-up study. J Am Coll Cardiol.41(2):239-244 (2003).
- Triedman JK. Management of asymptomatic Wolff-Parkinson-White syndrome. Heart.95(19):1628-1634 (2009).
- Niksch AL, Dubin AM. Risk stratification in the asymptomatic child with Wolff-Parkinson-White syndrome. Curr Opin Cardiol.21(3):205-207 (2006).
- Sarubbi B, D'Alto M, Vergara P, et al. Electrophysiological evaluation of asymptomatic ventricular pre-excitation in children and adolescents. Int J Cardiol.98(2):207-214 (2005).
- Chubb H, Campbell RM, Motonaga KS, et al. Management of asymptomatic Wolff-Parkinson-White pattern by pediatric electrophysiologists. J Pediatr.213:88-95 (2019).
- Gaita F, Giustetto C, Riccardi R, et al. Stress and pharmacologic tests as methods to identify patients with Wolff-Parkinson-White syndrome at risk of sudden death. Am J Cardiol.64(8):487-490 (1989).
- LaRocca TJ, Beyersdorf GB, Li W, et al. Comparison of electrophysiologic profiles in pediatric patients with incidentally identified pre-excitation compared with Wolff-Parkinson-White syndrome. Am J Cardio.124(3):389-395 (2019).
- Klein GJ, Gulamhusein SS. Intermittent pre-excitation in the Wolff-Parkinson-White syndrome. Am J Cardiol.52(3):292-296 (1983).
- Kiger ME, Mccanta AC, Tong S, et al. Intermittent versus persistent Wolff‐Parkinson‐White syndrome in children: Electrophysiologic properties and clinical outcomes. Pacing Clin Electrophysiol.39(1):14-20 (2016).
- Mah DY, Sherwin ED, Alexander ME, et al. The electrophysiological characteristics of accessory pathways in pediatric patients with intermittent pre-excitation. Pacing Clin Electrophysiol.36(9):1117-1122 (2013).
- Escudero CA, Ceresnak SR, Collins KK, et al. Loss of ventricular preexcitation during noninvasive testing does not exclude high-risk accessory pathways: A multicenter study of WPW in children. Heart Rhythm.17(10):1729-1737 (2020).
- Wackel P, Irving C, Webber S, et al. Risk stratification in Wolff‐Parkinson‐White syndrome: the correlation between noninvasive and invasive testing in pediatric patients. Pacing Clin Electrophysiol.35(12):1451-1457 (2012).
- Benson DW, Cohen MI. Wolff-Parkinson-White syndrome: Lessons learnt and lessons remaining. Cardiol Young.27(S1):S62-S67 (2017).
- Bromberg BI, Lindsay BD, Cain ME, et al. Impact of clinical history and electrophysiologic characterization of accessory pathways on management strategies to reduce sudden death among children with Wolff-Parkinson-White syndrome. J Am Coll Cardiol.27(3):690-695 (1996).
- Paul T, Guccione P, Garson Jr A, et al. Relation of syncope in young patients with Wolff-Parkinson-White syndrome to rapid ventricular response during atrial fibrillation. Am J Cardiol. 65(5):318-321 (1990).
- Lee PC, Hwang B, Tai CT, et al. The different electrophysiological characteristics in children with Wolff‐Parkinson‐White syndrome between those with and without atrial fibrillation. Pacing Clin Electrophysiol.27(2):235-239 (2004).
- Dubin AM, Collins KK, Chiesa N, et al. Use of electrophysiologic testing to assess risk in children with Wolff-Parkinson-White syndrome. Cardiol Young.12(3):248-252 (2002).
- Dubin AM, Jorgensen NW, Radbill AE, et al. What have we learned in the last 20 years? A comparison of a modern era pediatric and congenital catheter ablation registry to previous pediatric ablation registries. Heart Rhythm.16(1):57-63 (2019).
- Kugler JD, Danford DA, Houston KA, et al. Pediatric radiofrequency catheter ablation registry success, fluoroscopy time, and complication rate for supraventricular tachycardia: Comparison of early and recent eras. J Cardiovasc Electrophysiol.13(4):336-341 (2002).
- Etheridge SP, Gakenheimer-Smith L, Asaki SY, et al. Asymptomatic Wolff-Parkinson-White syndrome: An ounce of prevention is worth the risk of cure. Curr Cardiol Rep.25(6):543-551 (2023).
- Szafran E, Bartecki M, Bukowska-Posadzy A, et al. Do children with asymptomatic ventricular pre-excitation have similar quality of life as healthy children? Kardiol Pol.(2024).
- Obeyesekere MN, Klein GJ. The asymptomatic Wolff-Parkinson-White patient: Time to be more proactive? Circulation.130(10):805-807 (2014).
- Skanes AC, Obeyesekere M, Klein GJ, et al. Electrophysiology testing and catheter ablation are helpful when evaluating asymptomatic patients with Wolff-Parkinson-White pattern: The con perspective. Card Electrophysiol Clin.7(3):377-383 (2015).
- Pappone C, Santinelli V, Manguso F, et al. A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome. N Engl J Med.349(19):1803-1811 (2003).