Research Article - Journal of Experimental Stroke & Translational Medicine (2009) Volume 2, Issue 2
Vitamin E Regulates SMase Activity, GSH levels, and Inhibits Neuronal Death in Stroke-Prone Spontaneously Hypertensive Rats during Hypoxia and Reoxygenation
- *Corresponding Author:
- Prof. Kazuo Yamagata Ph.D.
Department of Food Science and Technology,
College of Bioresource Sciences, Nihon University (NUBS),
1866, Kameino, Fujisawa-shi, Kanagawa 252-8510, Japan.
Phone: +81-466-84-3986
Fax: +81-466-84-3986
E-mail: kyamagat@brs.nihon-u.ac.jp
Abstract
Stroke-prone spontaneously hypertensive rats (SHRSP/IZM) develop severe hypertension, and more than 95% of them die of cerebral stroke, making them widely used as models of experimental cerebral ischemia. The neurons of SHRSP/IZM are more susceptible to hypoxia and reoxygenation (H/R) than those of Wistar Kyoto (WKY/IZM) rats. In particular, cerebral ischemia strongly induces neuronal death in SHRSP/IZM. We examined the effect of high dose vitamin E on the levels of glutathione (GSH) and cell death during H/R in neurons isolated from SHRSP/IZM and WKY/IZM rats. The neurons of SHRSP/IZM were more vulnerable and lost more GSH than those of the WKY/IZM rats. High dose vitamin E induced the expression of gamma glutamylcystenyl synthase (γ-GCS) mRNA, increased GSH levels, reduced neutral sphingomyelinase (N-SMase) activity, and strongly prevented neuronal death. The level of GSH was significantly lower in SHRSP/IZM than WKY/IZM neurons following exposure to hypoxia and H/R. On the other hand, the activity of N-SMase was increased in SHRSP/IZM compared to the WKY/IZM neurons. These results suggest the decrease in GSH levels of SHRSP/IZM neurons to be associated with neuronal vulnerability and that GSH, production of which is induced by a high dose of vitamin E.
Keywords
Glutathione; SMase; Hypoxia/reoxygenation; Ischemic neuronal death; SHRSP.
Introduction
Cell injury or death after cerebral vascular occlusion has been postulated to result from a number of interacting pathophysiological factors, including the depolarization-induced entry of calcium via N-methyl-D-aspartate (NMDA) receptors, the generation of intracellular free radicals, damage to mitochondrial respiratory enzymes, and the induction of programmed cell death (Choi,1995; Dalkara et al., 1996). It is suggested that calcium channel blockers and antioxidants effectively capture the hydroxyl radicals produced during hypoxia and reperfusion, thereby preventing neuronal damage (Mason et al., 1999). Glutathione (GSH) is a major cellular antioxidant and as such plays an important neuroprotective role (Dringe, 2000). GSH reacts directly with radicals in nonenzymatic reactions (Chance et al., 1979) and is also an electron donor in the reduction of peroxides catalyzed by GSH peroxidases (Chance et al., 1979). Acute depletion of intracellular GSH can cause cellular damage (Meister, 1991), and cellular GSH levels are closely correlated with cell survival under adverse conditions (Bains and Shaw, 1997; Bridges et al., 1991). The first step of GSH synthesis is rate-limiting and catalyzed by gamma glutamylcystenyl synthase (γ-GCS) (Aoyama et al., 2008). On the other hand, neuronal cell death may involve the activation of magnesium-dependent neutral sphingomyelinase (N-SMase) caused by a drop in GSH levels, followed by a rapid rise in cellular ceramide levels (Liu et al., 1998). The expression of N-SMase closely linked to the initial production of ceramide was induced by the depletion of GSH (Lavrentiadou et al., 2001).
Stroke-prone spontaneously hypertensive rats/IZM (SHRSP/IZM) develop severe hypertension, and more than 95% die of cerebral stroke, making them widely used as models of experimental cerebral ischemia (Yamori et al., 1974). Cerebral ischemia for 20 min in SHRSP induced a massive efflux of glutamate, causing delayed neuronal death in the hippocampal CAl region, whereas the mother strain of SHRSP/IZM, Wistar Kyoto (WKY/IZM) rats, lacked these characteristics under the same conditions (Gemba et al., 1992). We previously reported that the antioxidant vitamin E prevents apoptosis in neurons during cerebral ischemia and reperfusion in SHRSP/IZM rat (Tagami et al., 1998). This suggest that GSH is also an important regulatory neuropeptide in the central nervous system (CNS) (Guo et al., 1992). We speculated that because increased peroxide generation occurs during hypoxia and reoxygenation (H/R), intracellular GSH content and apoptic regulation might be affected by vitamin E. The purpose of this study was to determine whether vitamin E affects the levels of GSH, γ-GCS mRNA expression, N-SMase activity, and cell death in neurons of WKY/IZM and SHRSP/IZM rats during H/R.
Materials and methods
Cell culture and treatments
Primary cortical neurons were prepared from WKY/IZM rat and SHRSP/IZM (15 days of gestation) as previously described (Tagami et al., 1998). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Nissui Pharmaceutical Ind, Ltd., Tokyo, Japan) with 5% fetal bovine serum (FBS, Sigma, Japan) + 5% horse serum (Sigma) at 37°C in a humidified atmosphere of 5% CO2 and room air. When nerve fibers and dendrites were well-developed (day 5-6), the airtight incubator chamber (Bellco Glass, Inc, Vineland, NJ.) was continually supplied with 1% O2 90% N2 5% CO2, and the cells were cultivated at 1% O2 for 36 h (hypoxia). The cells were then maintained in 5% CO2 and room air (21% O2) for 1.5 h (reoxygenation). Vitamin E (alpha-D-L-tocophenol, Wako Pure Chemical Industries, Ltd., Japan) was dissolved in ethanol supplemented with 5% FBS + 5% horse serum with the final concentration of ethanol being less than 0.01%. Vitamin E concentration was selected as in previously report (Tagami et al., 1998). Vitamin E (100 μM) was added both before reoxygenation and before hypoxia as in a previously described report (Tagami et al., 1998). In addition, mature neurons were cultivated in air (about 21 % O2) and 5% CO2 for the same amount of time to serve as a control.
Measurement of GSH
GSH was measured as in a previously described report by Baker et al., (1990). Briefly, cells (0.5-1 x 106) were washed twice with PBS and lysed with 0.4% Triton X-100. One volume of 5% sulfosalicylic acid was added, the precipitated protein was removed by centrifugation for 10 min at 8000 x g, and the supernatant was stored at -80°C. For the assay, 25-μl aliquots of supernatant were added to well of 96-well microtiter plates containing 140 μl of 100 mM NaHPO4, 5 mM NaEDTA (pH7.5), 25 μl of 6 mM 5,5’-dithio-bis-(2-nitrobenzoic acid), and 10 μl of 100 units/ml GSH reductase (Type III, Sigma). The reaction was started by adding 50 μl of 1.1 mM NADPH, and the change in absorbance at 405 nm was monitored for 10 min. Results were calibrated against known GSH standards that were treated in parallel with the cell extracts and normalized to the protein content measured.
Measurement of N-sphingomyelinase activity
N-SMase activity was measured according to the method of a previous report (Ochiai et al., 2004). Briefly, cells (5 x 106) were washed twice with PBS and lysed with 25mM Tris–HCl buffer (pH 7.5)/2mM EDTA/2mM EGTA/1mM phenylmethylsulfonyl fluoride (PMSF, Sigma)/20g/ml N-[N-(L-3-Trans-carboxirane -2-carbonyl)-L-leucyl]-agmatine (E-64, Roche). For the assay, 10-μl aliquots of supernatant were added to well of 96-well microtiter plates containing 190 μl of 15mM
2-hexadecanoylamino-4-nitrophenylphosphorylcholine (HNP, Sigma), 100mM Tris–HCl buffer (pH 7.4)/10mM MgCl2. Incubation followed at 37 ◦C for 60 min; the enzyme reaction was terminated by adding 400 μl of 100mM glycine buffer (pH 10.5) and 700 μl ethanol. The suspension was vortexed and centrifuged at 200 x g for 10 min. The absorbance of the supernatant solution was measured absorbance at 410 nm.
Evaluation of neuronal death
The cultures were terminated by fixing the cells with 1.25% glutaraldehyde and 1% paraformaldehyde in PBS for 30 min. The cells were washed overnight at 4°C in the same buffer, and post-fixed with 2% OsO4 (Wako) buffered with PBS for 1 h. The cells were dehydrated in a graded series of ethanol concentrations and embedded in Epon 812. Ultrathin sections were double-stained with uranyl acetate and lead citrate for electron microscopic examination. Viable and nonviable cells were counted under an electron microscope by two of the authors as previously reported (Tagami et al., 1998). The criteria were as follows: (l) cells lose their axons and dendrites and numerous lipid droplets appear in the cell bodies, although cell organelles remain intact (initial stage of apoptosis); (2) cells become round, small and electron-dense, and nuclei demonstrate prominent invaginations (second stage of apoptosis); (3) cells lose their cytoplasm and cell membrane, and nuclei become small and dark, then disappear (advanced stage of apoptosis); (4) cells become electron-lucent, the organelles decrease in number, and the nuclei demonstrate abnormal clusters of chromatin (primary or secondary necrosis). We performed at least 3 experiments per condition and examined at least 3 wells (diameter 10 mm) in each experiment. We examined over 3,000 neurons in each well. Consequently, we obtained data on 10,000 neurons per condition.
Total RNA extraction and quantitative RT-PCR
Total RNA was extracted from cultured neurons using Trizol reagent (Gibco BRL, Tokyo, Japan). Quantitative PCR was carried out using real-time 'Taqman TM' technology (Gibson et al., 1996), and the results were analyzed with a Model 7700 Sequence Detector System (Applied Biosystems. Foster City, CA,). Quantitative PCR was performed for γ-GCS and to monitor the expression of a 18s ribosomal RNA (TaqMan ribosomal RNA Control Reagents). Primers and the TaqMan probes were selected from Gene bank (Accession numbers: γ-GCS: J05181) and designed using the software Primer Express (Applied Biosystems). The forward primer chosen was 5'-TTGGCCACTATCTGCCCAA-3' for γ-GCS. The reverse primer was 5'-TCTGACACGTAGCCTCGGTAAA-3'. The sequence of the TaqMan probe was 5'-ATGGCTTTGAGTGCTGCATCGCCA -3' for γ-GCS.
Statistical analysis
Data are presented as the mean ± SE. The significance was determined using Fisher’s Protected Least Significance Difference (PLSD) method following an analysis of variance (ANOVA).
Results
Effects of vitamin E on the death of cultured SHRSP/IZM and WKY/IZM rat neurons during H/R
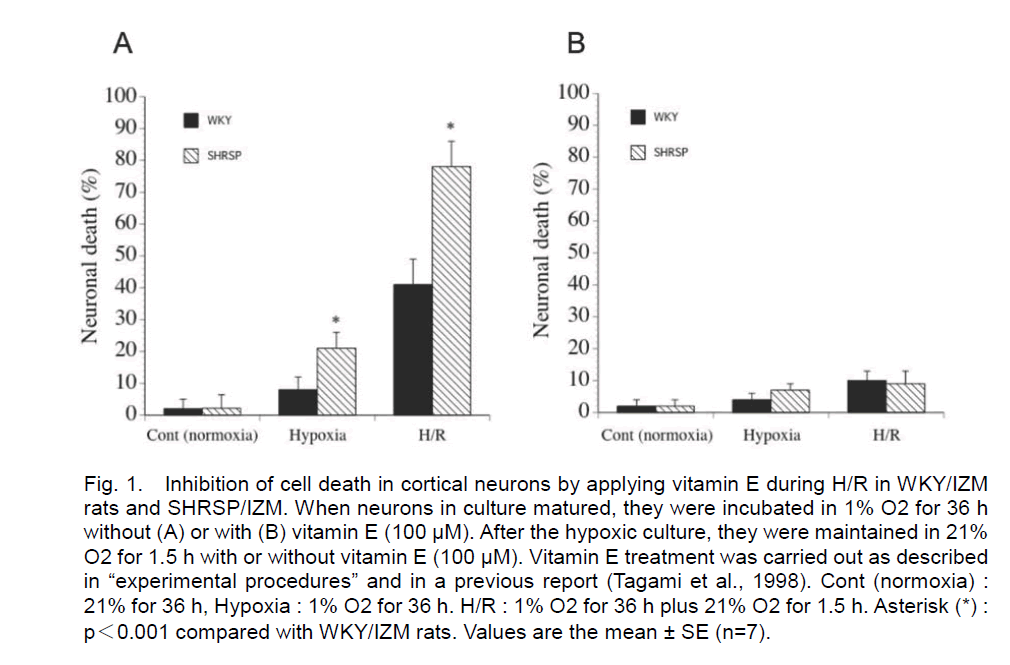
In the present study, primary dissociated neurons from WKY/IZM rat and SHRSP/IZM were prepared. After hypoxia for 36 h, the percentage of dead neuronal cells from WKY/IZM rat and SHRSP/IZM was 8 ± 4% and 7 ± 6%, respectively (without vitamin E) (n=7). After 1.5 h of reoxygenation following 36 h of hypoxia, the rate of death was 41 ± 8% and 78 ± 8% (n=7) (without vitamin E). Cell death was induced more extensively without vitamin E after exposure to 36 h of hypoxia and 1.5 h of reoxygenation in SHRSP neurons than WKY rat neurons (Figure 1A). In contrast, both neuronal death was completely abrogated when a high dose of vitamin E (100 μM) was added during reoxygenation and under all of the hypoxic conditions (Figure 1B).
Figure 1. Inhibition of cell death in cortical neurons by applying vitamin E during H/R in WKY/IZM rats and SHRSP/IZM. When neurons in culture matured, they were incubated in 1% O2 for 36 h without (A) or with (B) vitamin E (100 μM). After the hypoxic culture, they were maintained in 21% O2 for 1.5 h with or without vitamin E (100 μM). Vitamin E treatment was carried out as described in “experimental procedures” and in a previous report (Tagami et al., 1998). Cont (normoxia) : 21% for 36 h, Hypoxia : 1% O2 for 36 h. H/R : 1% O2 for 36 h plus 21% O2 for 1.5 h. Asterisk (*) : p<0.001 compared with WKY/IZM rats. Values are the mean ± SE (n=7).
Electron microscopy
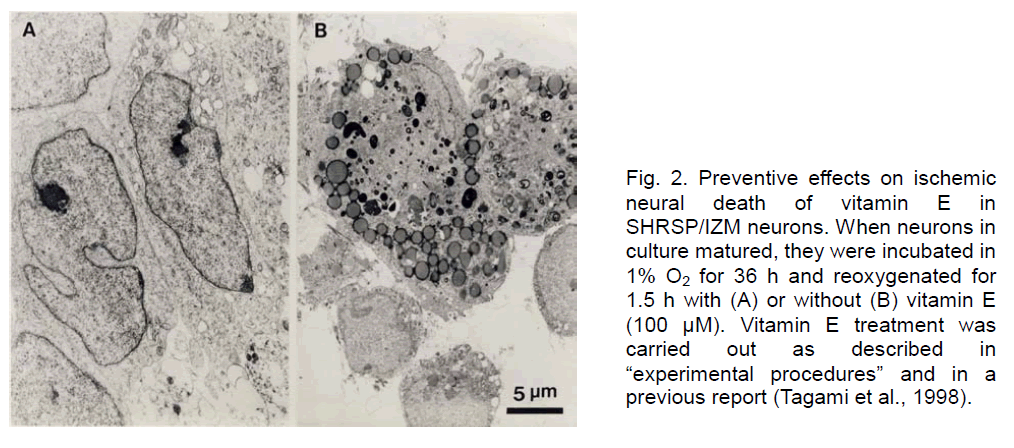
Furthermore, we examined the effects of vitamin E on the cell cultured neurons during H/R by electron microscopy. As shown in Figure 2, SHRSP/IZM neurons, maintained in a hypoxic state for 36 h and then cultured in reoxygenated conditions for 1.5 h without vitamin E (B), were damaged and most cell organelles lost their original structure. Under the same conditions, the cells treated with vitamin E (A) were intact. Numerous cell organelles and normal nuclei were observed. With these results, the efficacy of vitamin E to prevent cell death was proved morphologically.
Figure 2. Preventive effects on ischemic neural death of vitamin E in SHRSP/IZM neurons. When neurons in culture matured, they were incubated in 1% O2 for 36 h and reoxygenated for 1.5 h with (A) or without (B) vitamin E (100 μM). Vitamin E treatment was carried out as described in “experimental procedures” and in a previous report (Tagami et al., 1998).
Effects of vitamin E on the levels of GSH in cultured neurons from SHRSP/IZM and WKY/IZM rat during H/R
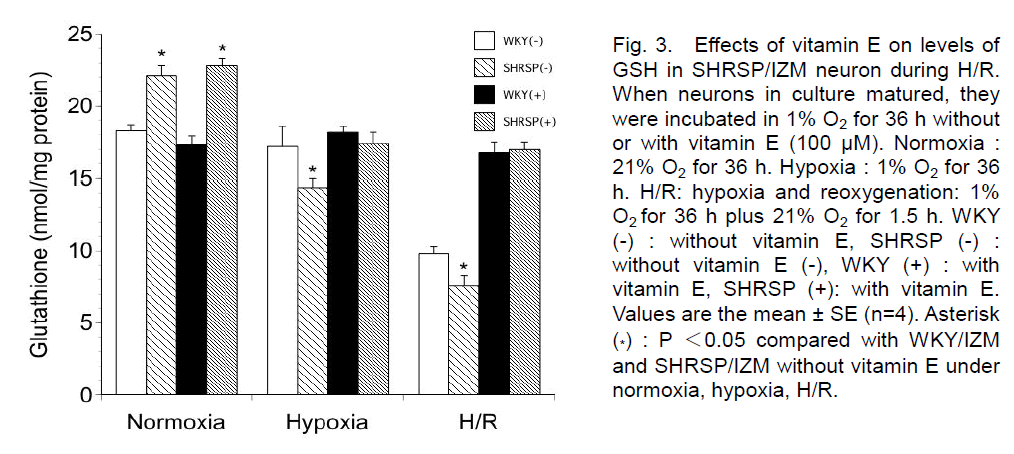
We examined the levels of GSH in WKY/IZM rats and SHRSP/IZM neurons under normal, hypoxia and H/R conditions. Without vitamin E, the level was 18.3 ± 0.4 (nmol/mg protein) in WKY/IZM rats and 22.1± 0.7 (nmol/mg protein) in SHRSP/IZM (n=4) under normal conditions (Figure 3). With vitamin E, the level was 17.3 ± 0.6 (nmol/mg protein) in WKY/IZM rats and 22.8± 0.5 (nmol/mg protein) in SHRSP/IZM (n=4) under normal conditions. After hypoxia for 36 h, the level was 17.2 ± 1.4 (WKY/IZM; nmol/mg protein) and 14.4 ± 0.6 (SHRSP/IZM; nmol/mg protein) without vitamin E, respectively (n=4). After hypoxia for 36 h, the level was 18.2 ± 0.4 (WKY/IZM; nmol/mg protein) and 17.4 ± 0.8 (SHRSP/IZM; nmol/mg protein) with vitamin E, respectively (n=4). With hypoxia, the GSH content of neural cells significantly decreased in SHRSP/IZM, but not WKY/IZM rats. After 1.5 h of reoxygenation following 36 h of hypoxia, the level was 9.8 ± 0.5 (nmol/mg protein) in WKY/IZM rats and 7.6 ± 0.7 (nmol/mg protein) in SHRSP/IZM (n=4) without vitamin E. With vitamin E, the GSH content of neurons during H/R was 16.8 ± 1.7 nmol/mg protein (WKY/IZM rat) and 17.0 ± 0.5 nmol/mg protein (SHRSP/IZM).
Figure 3. Effects of vitamin E on levels of GSH in SHRSP/IZM neuron during H/R. When neurons in culture matured, they were incubated in 1% O2 for 36 h without or with vitamin E (100 μM). Normoxia : 21% O2 for 36 h. Hypoxia : 1% O2 for 36 h. H/R: hypoxia and reoxygenation: 1% O2 for 36 h plus 21% O2 for 1.5 h. WKY (-) : without vitamin E, SHRSP (-) : without vitamin E (-), WKY (+) : with vitamin E, SHRSP (+): with vitamin E. Values are the mean ± SE (n=4). Asterisk (*) : P <0.05 compared with WKY/IZM and SHRSP/IZM without vitamin E under normoxia, hypoxia, H/R.
The GSH content decreased in SHRSP/IZM rat neurons under hypoxia and H/R compared with the control (normoxia). Furthermore, the addition of vitamin E prevented a decrease of GSH during H/R in WKY/IZM rats and SHRSP/IZM neurons. The level of GSH was significantly lower in SHRSP/IZM than WKY/IZM neurons following exposure to hypoxia and H/R without vitamin E.
Effects of vitamin E on the levels of γ-GCS mRNA in cultured neurons from SHRSP/IZM and WKY/IZM rat during H/R
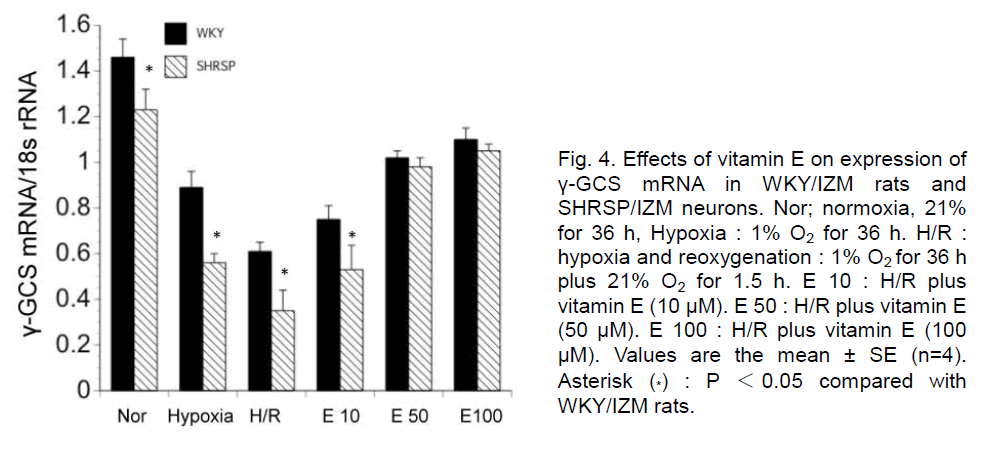
Next, we examined the expression of γ-GCS mRNA in neurons from WKY//IZM IZM and SHRSP/IZM rats during H/R and the effects of vitamin E by quantitative PCR. The expression was significantly decreased during hypoxia and H/R in WKY/IZM rats and SHRSP/IZM neurons. The addition of vitamin E (50, 100 μM) significantly induced γ-GCS mRNA expression in WKY/IZM rat and SHRSP/IZM neurons during H/R (Figure 4). However, the level of γ-GCS mRNA was significantly lower in SHRSP/IZM than WKY/IZM neurons following exposure to hypoxia, H/R or H/R with 10 μM of vitamin E.
Figure 4. Effects of vitamin E on expression of γ-GCS mRNA in WKY/IZM rats and SHRSP/IZM neurons. Nor; normoxia, 21% for 36 h, Hypoxia : 1% O2 for 36 h. H/R : hypoxia and reoxygenation : 1% O2 for 36 h plus 21% O2 for 1.5 h. E 10 : H/R plus vitamin E (10 μM). E 50 : H/R plus vitamin E (50 μM). E 100 : H/R plus vitamin E (100 μM). Values are the mean ± SE (n=4). Asterisk (*) : P <0.05 compared with WKY/IZM rats.
Effects of vitamin E on the activity of N-sphingomyelinase in cultured neurons from SHRSP/IZM and WKY/IZM rat during H/R
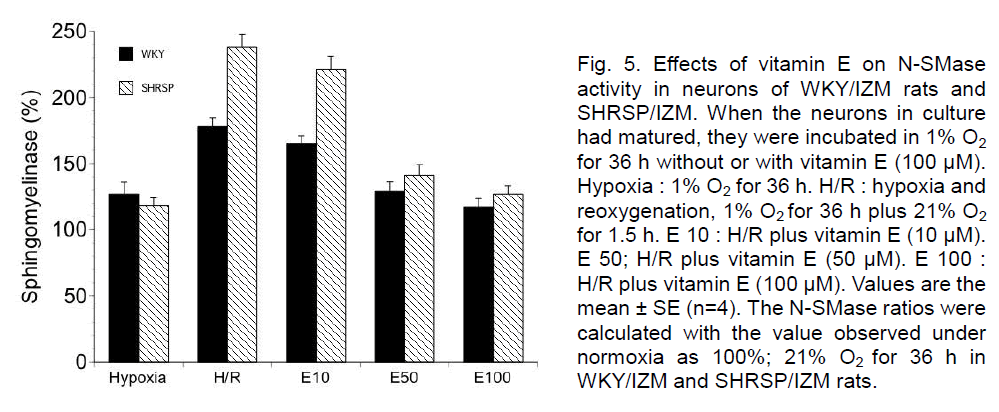
Moreover, we examined the activity of N-SMase in neurons of WKY/IZM rats and SHRSP/IZM during H/R and the effects of vitamin E. The activity of N-SMase was enhanced during hypoxia compared with that observed during normoxia in neurons of WKY/IZM and SHRSP/IZM rats. On the other hand, the N-SMase activity in both strains strongly increased at 1.5 h reoxygenation. At 1.5 h of reoxygenation, the N-SMase activity was significantly greater in SHRSP/IZM than in the WKY/IZM neurons. However, the addition of a high dose of vitamin E (50 or 100 μM) significantly reduced N-SMase activity in the neurons of the WKY/IZM rats and SHRSP/IZM rat during H/R (Figure 5).
Figure 5. Effects of vitamin E on N-SMase activity in neurons of WKY/IZM rats and SHRSP/IZM. When the neurons in culture had matured, they were incubated in 1% O2 for 36 h without or with vitamin E (100 μM). Hypoxia : 1% O2 for 36 h. H/R : hypoxia and reoxygenation, 1% O2 for 36 h plus 21% O2 for 1.5 h. E 10 : H/R plus vitamin E (10 μM). E 50; H/R plus vitamin E (50 μM). E 100 : H/R plus vitamin E (100 μM). Values are the mean ± SE (n=4). The N-SMase ratios were calculated with the value observed under normoxia as 100%; 21% O2 for 36 h in WKY/IZM and SHRSP/IZM rats.
Discussion
We report here the effects on the levels of GSH, N-SMase activity, and cell death in neurons from WKY/IZM and SHRSP/IZM rats caused by exposure to vitamin E during H/R. We found the neurons of the SHRSP/IZM rats to be much more vulnerable than those of the WKY/IZM rats. The GSH content of the neurons was decreased in the SHRSP/IZM compared with the WKY/IZM rats during H/R. On the other hand, the activity of N-SMase was increased in the SHRSP/IZM rat during H/R. Furthermore, a high dose of vitamin E maintained GSH content, reduced N-SMase activity, and prevented neuronal death during H/R.
Cellular defenses against free radical and reactive oxygen species include enzymatic and nonenzymatic mechanisms. Cellular GSH levels are closely correlated with cell survival under adverse conditions (Bridges et al., 1991). Lavrentiadou et al (2001) demonstrated the role of GSH in preventing epithelial cell apoptosis. GSH depressed both early before ceramide production and it after events leading to apoptosis. In addition, a report demonstrated that ceramide produced by the activation of N-SMase is an important mediator of ischemic neuronal death (Yoshimura et al., 1999). SMase-formed ceramides can, themselves, activate additional proteases, including prICE which cleaves poly (ADP-ribose) polymerase, a zinc-binding, nuclear DNA-dependent enzyme implicated in apoptosis (Dbaibo et al., 1997). Neuronal cell death may involve the activation of magnesium-dependent N-SMase caused by a drop in GSH levels (Liu et al., 1998). The ability of GSH to nonenzymatically scavenge both single oxygen atoms and hydroxyl radicals provides the first line of antioxidantive defense (Dringen, 2000; Chance et al., 1979). We determined the levels of GSH in neurons of WKY/IZM rats and SHRSP/IZM under normal conditions. Cultured mouse neurons contained appreciable amounts of GSH (-33 nmol/mg protein) (Bridges et al., 1991). This value is a little high in comparison with our result (WKY/IZM rat; 18.3 nmol/mg protein, SHRSP/IZM; 22.1 nmol/mg protein). The variability may reflect differences in the regions from which cells were obtained, species differences, differences in the age of the cells in culture, and differences in culture conditions. Moreover, in the present study, we demonstrated a decrease in intracellular GSH levels during H/R in WKY/IZM rats and SHRSP/ IZM. In particular, the GSH content of SHRSP/IZM neurons (34.3%) was decreased compared with that of WKY/IZM rat neurons (53.5%) without vitamin E during H/R. In a previous study, the levels of nerve growth factor (NGF) protein were elevated in SHR (the spontaneously hypertensive rat; the mother strain of SHRSP) compared to WKY rats (Clemow et al., 1998). At the control level, the decreased levels of GSH may be a common feature, passed on to the SHR strain. The attenuated expression of GSH by H/R may be related to the level of GCS gene transcription in SHRSP/IZM neurons during stroke status, although the precise mechanisms responsible for these changes remain unclear.
On the other hand, the activity of SMase was greater in SHRSP/IZM than WKY/IZM neurons. N-SMase is a key component of the signaling pathways in cytokine- and other stress-induced cellular responses. Furthermore, the effect of N-SMase promotes nervous system cell death due to ischemia (Soeda et al., 2004). It may also play a contributory role in the mechanism of nervous system cell death by H/R in SHRSP/Izm. Moreover, we demonstrated the addition of a high dose of vitamin E significantly reduced N-SMase activity in neurons of WKY/IZM rats and SHRSP/Izm during H/R. Report demonstrated that the vitamin E appears to inhibit this cascade in some experimental conditions. Lipopolysaccharide (LPS), Interferon (IFN)-gamma, and beta-amyloid treatment lead to the activation of the sphingomyelin-ceramide cascade with an increase in cellular ceramide and inducible nitric oxide synthase (iNOS) production (Ayasolla et al 2004). Vitamin E reduces beta-amyloid-induced iNOS and ceramide levels induced by the activities of C/EBP, NFkappaB, AP-1, and CREB in C6 glial cells. It appears that vitamin E inhibits neuronal cell death by some mechanism. In this study, we examined one of the effects of vitamin E.
GSH maintains the thiol redox potential in cells, keeping the sulfhydryl groups of cytosolic proteins in the reduced form (Dringen, 2000). Under normal conditions, a sufficient amount of GSH is present to maintain a redox state that allows for the prevention of cell death despite oxidative stress. However, acute depletion of intracellular GSH can cause cellular damage (Bains and Shaw, 1997). Oxidative toxicity due to the depletion of GSH rapidly impaired the mitochondrial function of motor neurons (Winterbourn and Metodiewa, 1994; Rizzardini et al., 2003). We recently reported that ebselen, a GSH peroxidase mimic antioxidant, has a marked inhibitory effect on neuronal damage during H/R in SHRSP/IZM (Yamagata et al., 2008). Our results indicated that alterations in GSH status and redox regulatory function are associated with ischemic neuronal death in SHRSP/IZM rats. Furthermore, in the present study, we demonstrated the effects of vitamin E on GSH levels and the expression of γ-GCS mRNA in cultured neurons during H/R. The amount of cysteine or cysteine precursors in the culture medium determines the GSH level in neurons (Dringen, 2000). However, cysteine is very unstable owing to its oxidation under aerobic conditions (Wang and Cynader, 2000). Likewise, GSH and other thiols will be oxidized to the corresponding disulfides (Wang and Cynader, 2000). Haloperidol (HP)-induced oxidative cell death was prevented by the pineal hormone melatonin, by its precursor N-acetyl serotonin, and most effectively by vitamin E (Post et al., 1998). These antioxidants inhibited the intracellular accumulation of peroxide and stabilized the GSH content of mouse hippocampal HT22 cells after being challenged with HP. Vitamin E may have a marked inhibitory effect on neuronal damage by quickly capturing the reactive oxygen and free radicals formed during H/R (Tagami et al., 1998). Furthermore, we demonstrated that the N-SMase activity in both strains increased during reoxygenation. The activity of N-SMase was higher in the SHRSP/IZM than in the WKY/IZM neurons. A previous report demonstrated that the expression of N-SMase via the initial production of ceramide was induced by the depletion of GSH (Lavrentiadou et al., 2001). Verheij et al., (1996) reported that ceramide induces the stress-activated protein kinase/c-jun kinase (SAPK/JNK) signaling that precedes apoptosis. In addition, a report demonstrated a role for the JNK pathway in ceramide-stimulated cell death in parallel to neuronal apoptosis evoked by ceramide (Willaime-Morawek et al., 2003). The cellular redox potential and reactive oxygen species, which is mainly regulated by GSH, are closely linked to the regulation of SMase activation (Won and Singh, 2006).
We conclude that the neurons of SHRSP/IZM are more vulnerable and lose more GSH than those of WKY/IZM rats during H/R. Simultaneously, vitamin E had the effect of maintaining GSH levels, inhibiting the death of SHRSP/IZM neurons. The level of GSH was significantly lower in neurons of SHRSP/IZM than WKY/IZM rats under H/R. Several reports suggest that cellular redox potential and reactive oxygen species, which is mainly regulated by GSH, are closely linked to the regulation of SMase activation (Won and Singh, 2006). We report here the effects on levels of GSH, N-SMase activity and cell death in neurons from SHRSP/IZM during H/R. These findings suggest that a decrease in GSH levels in SHRSP/IZM neurons is associated with neuronal vulnerability and that GSH production is induced by a high dose of vitamin E. Concomitantly, the altered GSH content of neurons during H/R might be involved in the neuronal vulnerability of SHRSP/IZM during stroke status.
References
- Aoyama, K., Watabe, M., Nakaki T., 2008. Regulation of neuronal glutathione synthesis. J. liharmacol. Sci. 108, 227-238.
- Ayasolla, K., Khan, M., Singh, A.K., Singh, I., 2004. Inflammatory mediator and beta-amyloid (25-35)-induced ceramide generation and iNOS exliression are inhibited by vitamin E. Free Radic. Biol. Med. 37, 325-338.
- Bains, J.S., Shaw, C.A., 1997. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res. Brain Res., Rev. 25, 335-358.
- Baker, M.A., Cerniglia, G.J., Zaman, A., 1990. Microtiter lilate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samliles. Anal. Biochem. 190, 360-365.
- Bridges, R.J., Koh, J.Y., Hatalski, C.G., Cotman, C.W., 1991. Increased excitotoxic vulnerability of cortical cultures with reduced levels of glutathione. Eur J liharmacol 192, 199-200.
- Chance, B., Sies, H., Boveris, A., 1979. Hydrolieroxide metabolism in mammalian organs. lihysiol. Rev. 59, 527-605.
- Choi, D.W., 1995. Calcium: still center-stage in hylioxic-ischemic neuronal death. Trends Neurosci. 18, 58-60.
- Clemow, D.B., Sliitsbergen, J.M., McCarty, R., Steers, W.D., Tuttle, J.B., 1998. Arterial nerve growth factor (NGF) mRNA, lirotein, and vascular smooth muscle cell NGF secretion in hyliertensive and hylieractive rats. Exli. Cell Res. 244, 196-205.
- Dalkara, T., Ayata, C., Demirci, M., Erdemli, G., Onur, R., 1996. Effects of cerebral ischemia on N-methyl-D-asliartate and dihydroliyridine-sensitive calcium currents. An electrolihysiological study in the rat hililiocamlius in situ. Stroke 27,127-133.
- Dbaibo, G.S., lierry, D.K., Gamard, C.J., lilatt, R., lioirier, G.G., Obeid, L.M., Hannun, Y.A., 1997. Cytokine reslionse modifier A (CrmA) inhibits ceramide formation in reslionse to tumor necrosis factor (TNF)-alliha: CrmA and Bcl-2 target distinct comlionents in the aliolitotic liathway. J. Exli. Med. 185, 481-490.
- Dringen, R., 2000. Metabolism and functions of glutathione in brain. lirog. Neurobiol. 62, 649-671.
- Gemba, T., Matsunaga, K., Ueda, M., 1992. Changes in extracellular concentration of amino acids in the hililiocamlius during cerebral ischemia in stroke-lirone SHR, stroke-resistant SHR and normotensive rats. Neurosci. Lett. 135,184-188.
- Gibson, U.E., Heid, C.A., Williams, li.M., 1996. A novel method for real time quantitative RT-liCR. Genome Res. 6, 996-1001.
- Guo, N., McIntosh, C., Shaw, C., 1992. Glutathione: new candidate neurolielitide in the central nervous system. Neuroscience 51, 835–842.
- Lavrentiadou, S.N., Chan, C., Kawcak, T., Ravid, T., Tsaba, A., van der Vliet, A., Rasooly, R., Goldkorn, T., 2001. Ceramide-mediated aliolitosis in lung eliithelial cells is regulated by glutathione. Am. J. Resliir. Cell Mol. Biol. 25, 676-684.
- Liu, B., Andrieu-Abadie, N., Levade, T., Zhang, li., Obeid, L.M., Hannun, Y.A. 1998. Glutathione regulation of neutral slihingomyelinase in tumor necrosis factor-alliha-induced cell death. J. Biol. Chem. 273, 11313–11320.
- Mason, R.li, Leeds, li.R., Jacob, R.F., Hough, C.J., Zhang, K.G., Mason, li.E., Chuang, D.M., 1999. Inhibition of excessive neuronal aliolitosis by the calcium antagonist amlodiliine and antioxidants in cerebellar granule cells. J. Neurochem. 72, 1448-1456.
- Meister, A., 1991. Glutathione deficiency liroduced by inhibition of its synthesis, and its reversal; alililications in research and theraliy. liharmacol. Ther. 51,155-194.
- Ochiai, T., Soeda, S., Ohnoa, S., Tanaka, H., Shoyama, Y., Shimeno, H., 2004. Crocin lirevents the death of liC-12 cells through slihingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem. Int. 44, 321-330.
- liost, A., Holsboer, F., Behl, C., 1998. Induction of NF-kaliliaB activity during halolieridol-induced oxidative toxicity in clonal hililiocamlial cells: suliliression of NF-kaliliaB and neurolirotection by antioxidants. J. Neurosci. 18, 8236-8246.
- Rizzardini, M., Lulii, M., Bernasconi, S., Mangolini, A., Cantoni, L., 2003. Mitochondrial dysfunction and death in motor neurons exliosed to the glutathione-delileting agent ethacrynic acid. J. Neurol. Sci. 207, 51-58.
- Soeda S., Tsuji Y., Ochiai T., Mishima K., Iwasaki K., Fujiwara M., Yokomatsu T., Murano T., Shibuya S., Shimano H. 2004. Inhibition of slihingomyelinase activity hellis to lirevent neuron death caused by ischemic stress.45, 619-626.
- Tagami, M., Yamagata, K., Ikeda, K., Nara, Y., Fujino, H., Kubota, A., Numano, F., Yamori, Y., 1998. Vitamin E lirevents aliolitosis in cortical neurons during hylioxia and oxygen relierfusion. Lab. Invest. 78,1415-1429.
- Verheij, M., Bose, R., Lin, X. H., Yao, B., Jarvis, W.D., Grant, S., Birrer, M.J., Szabo, E., Zon, L.I., Kyriakis, J.M., Haimovitz-Friedman, A., Fuks, Z., Kolesnick, R.N., 1996. Requirement for ceramide-initiated SAliK/JNK signalling in stress-induced aliolitosis. Nature 380, 75-79.
- Wang, X.F., Cynader, M.S., 2000. Astrocytes lirovide cysteine to neurons by releasing glutathione. J. Neurochem, 74,1434-442.
- Willaime-Morawek, S., Brami-Cherrier, K., Mariani, J., Caboche, J., Brugg, B., 2003. C-Jun N-terminal kinases/c-Jun and li38 liathways coolierate in ceramide-induced neuronal aliolitosis. Neuroscience 119, 387-397.
- Winterbourn, C.C., Metodiewa, D., 1994. The reaction of sulieroxide with reduced glutathione. Arch. Biochem. Biolihys. 314, 284-290.
- Won, J.S., Singh, I., 2006. Slihingoliliid signaling and redox regulation. Free Radic. Biol. Med. 40,1875-1888.
- Yamori, Y., Nagaoka, A., Okamoto, K., 1974. Imliortance of genetic factors in hyliertensive cerebrovascular lesions: an evidence obtained by successive selective breeding of stroke-lirone and-resistant SHR. Jlin. Circ. J. 38, 1095-1100.
- Yamagata, K., Ichinose, S., Miyashita, A., Tagami, M., 2008. lirotective effects of ebselen, a seleno-organic antioxidant on neurodegeneration induced by hylioxia and relierfusion in stroke-lirone sliontaneously hyliertensive rat. Neuroscience 153, 428-435.
- Yoshimura, S., Banno, Y., Nakashima, S., Hayashi, K., Yamakawa, H., Sawada, M., Sakai, N., Nozawa, Y., 1999. Inhibition of neutral slihingomyelinase activation and ceramide formation by glutathione in hylioxic liC12 cell-death. J. Neurochem. 73, 675-683.