Perspective - Imaging in Medicine (2012) Volume 4, Issue 5
What are the risks of ultrasound and MRI to the fetus?
Magdalena Maria Woźniak*Department of Paediatric Radiology, Medical University of Lublin, Al. Raclawickie 1, 20–059 Lublin, Poland
- Corresponding Author:
- Magdalena Maria
Woźniak
Department of Paediatric Radiology
Medical University of Lublin
Al. Raclawickie 1, 20–059 Lublin, Poland
E-mail: mwozniak@hoga.pl
Abstract
Both ultrasound and MRI are recognized as generally safe imaging modalities and thus appropriate to be used in the diagnostics of the pregnant females and fetuses. However, to some extent, both techniques may carry some risks when used during pregnancy. To keep the fetus safe, knowledge of potential bioeffects is mandatory, as is understanding the background and the influence of particular factors for safety.
Keywords
fetal MRI ▪ fetal ultrasound ▪ imaging in pregnancy ▪ MRI safety ▪ ultrasound safety
Fetal ultrasound
“Diagnostic ultrasound studies of the fetus are generally considered safe during pregnancy,” reports Practice Guideline for the Performance of Obstetric Ultrasound Examinations published by the American Institute of Ultrasound in Medicine (AIUM) in 2007. On the other hand, there are numerous publications warning that, “although diagnostic ultrasound has had an excellent safety record – laboratory studies in animals have shown serious harm if the intensity is sufficiently high” [1]. In a review of epidemiologic studies of human exposure to ultrasound, there were no effects noted on childhood cancer, dyslexia, speech development or congenital anomalies [2]. However, there is very limited evidence that the frequent exposure of the human fetus to ultrasound waves may be associated with a nonsignificant decrease in newborn body weight [3], a reduction in the frequency of righthandedness [4–6] and delayed speech [7]. What is the truth? Is fetal ultrasound totally safe, as generally considered, or do we need to be careful not to harm the fetus? The question still seems to be particularly important facing the number of fetal ultrasound scans performed worldwide [8,9]. Assuming that it was only in 2010 that the number of births all over the world was approximately 130,000,000, and in the developed countries approximately 80% of pregnant females undergo at least one ultrasound scan in pregnancy, the scale is undisputable.
What kind of potential risks to the fetus may be carried by ultrasound? Ultrasound scans may induce adverse effects by either thermal or nonthermal means. For the end user, the indices providing information on potential adverse effects are the thermal index (TI), which gives some indication of potential temperature increase and thus thermal effects, and the mechanical index (MI), which gives an indication of potential for nonthermal (i.e., mechanical) effects [10].
■ Thermal effects
Thermally induced teratogenesis has been shown in many animal studies [11–15], as well as a few controlled human studies [16,17]. Ultrasound increases temperature in the focal area of the beam and, therefore, has the potential to cause thermal changes in tissue. Hyperthermia may cause a wide range of structural and functional defects, it is a recognized teratogen in mammalian laboratory animals and is a suspected teratogen for humans [18–21]. The human embryo and fetus may be especially vulnerable to elevated temperatures. As a general rule, maternal core body temperature increases above normal by 2°C for extended periods of time, by 2–2.5°C for 0.5–1 h and by 4°C for 15 min. This has resulted in developmental abnormalities in animal models. However, significant differences in thermoregulation and thermoneutral ambient temperatures make direct extrapolation of animal data to humans challenging, and the above temperatures may or may not be reasonable threshold predictions for adverse developmental effects in humans [1]. Using a pulsed spectral Doppler may result in local temperature increase, because exposure to pulsed spectral Doppler ultrasound can generate much higher levels of acoustic energy than B-mode, and thus significantly heat biologic tissue (spatial peak temporal average intensity = 1180 mW/cm2 in pulsed spectral Doppler compared with spatial peak temporal average intensity = 34 mW/cm2 in B-mode). It seems particularly important as this kind of scan requires the beam to remain stationary during examinations for longer periods. This may potentially have significant implications for sensitive neural tissue, such as that exposed during spectral Doppler flow studies of fetal cerebral vessels. The risk of inducing thermal effects is greater in the second and third trimesters, when fetal bone is intercepted by the ultrasound beam.
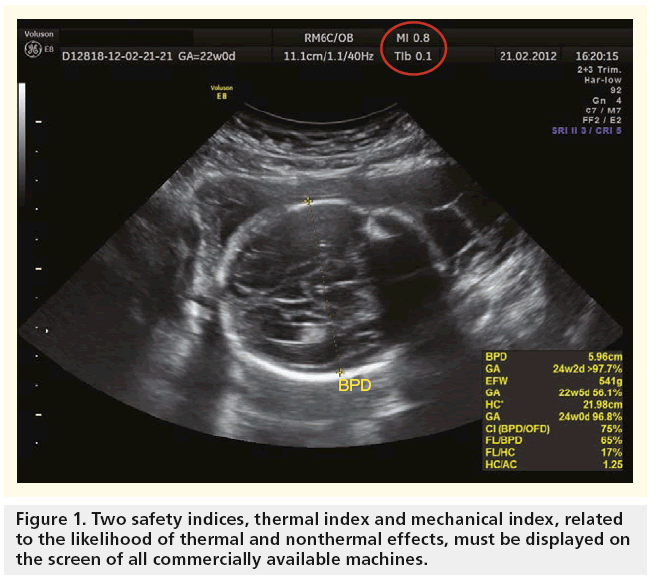
To allow clinical users of an ultrasound to understand the potential thermal bioeffects, standard indices, such as TI, provide quantitative safety-related information. The TI is the ratio of the power used to that required to produce a temperature rise of 1°C. It needs to be made very clear that TI does not represent an actual or an assumed temperature increase. It is the ratio of instantaneous power to the power theoretically needed to raise the tissue temperature by 1°C [10]. The TI has three variants:
▪ TI in soft tissue – to be used mostly in early pregnancy when ossification is low;
▪ TI in bone – to be used when the ultrasound beam impinges on bone, at or near the beam focus, such asin the late second and third trimesters of pregnancy;
▪ TI in the cranium – for transcranial studies when the transducer is essentially against bone, mostly for examinations in adult patients.
■ Nonthermal effects
Nonthermal adverse effects of ultrasound include acoustic cavitation, radiation force and acoustic streaming, and may be more significant in early gestation when the relatively loosely tethered embryonic tissues are exposed to an ultrasound beam in a liquid path. The likelihood of producing cavitation-type nonthermal effects is enhanced by the presence in the sound-field of gas-encapsulated echo-contrast media. Thus, ultrasound contrast agents are not licensed in pregnancy [22]. Nonthermal damage has been demonstrated in mammalian tissues containing gas. An MI value of 0.3 represents the threshold for the possibility of capillary bleeding in gas-containing organs, such as the lungs and intestines, as taken from the 1992 Statement on Nonhuman Mammalian in vivo Biological Effects of the AIUM 1993. An MI value of 0.7 is chosen as the threshold for cavitation, following the theoretical study by Apfel and Holland in 1991 [23], from which the formula for MI is derived [24].
■ The output display standard
The acoustic output (‘energy’) of ultrasound of commercially available ultrasound devices in clinical practice for fetal scanning used to be limited to 46 mW/cm2, but was subsequently increased to 94 mW/cm2 around 1986, and then to 720 mW/cm2 in 1993 by the US FDA. Since that time, all machines have to display two safety indices on the screen that relate to the likelihood of thermal and nonthermal effects (Figure 1). The operators should continually monitor their values and use control settings that keep them as small as is consistent with achieving diagnostically useful results. There should also be independent checks that the displayed TI and MI values are accurate [24].
■ How to keep fetal ultrasound safe?
There are a few major rules needed in order to keep the fetal ultrasound safe. First of all, the TI must be kept below 1. If TI ranges from 0.7 to 1, recommended maximum scanning time for obstetrical scanning may even last up to 60 min, which should usually be long enough to obtain relevant clinical information, even in complicated fetal anomalies. However, increasing TI to 2–2.5 decreases recommended maximum time of scanning to less than 4 min [24]. Secondly, the lowest possible output for the shortest possible time compatible with obtaining diagnostic information must be used. And thirdly, the exposure time must be limited to be as short as possible, according to the As Low As Reasonably Achievable (ALARA) principle. Particular care should be taken to reduce the risk of thermal hazard when exposing an 8-week-old embryo and the head, brain or spine of any fetus to diagnostic ultrasound [24]. However, the most important rule seems to be awareness of doctors and sonographers, who should realize how to translate technical aspects of ultrasound into potential bioeffects.
■ Nonmedical use
Nonmedical use of fetal ultrasound is also a subject worth mentioning, as it is recently becoming more and more popular all over the world. The International Society of Ultrasound in Obstetrics and Gynecology disapproves of the use of ultrasound for the sole purpose of providing souvenir images of the fetus [25,26] Also, AIUM strongly discourages the nonmedical use of ultrasound for psychosocial or entertainment purposes and strictly points out that the use of 2D/3D/4D ultrasound to only view the fetus, obtain a picture of the fetus or determine the fetal gender without a medical indication is inappropriate and contrary to responsible medical practice. However, the ‘business’ of nonmedical fetal ultrasound still seems not only to grow, but also to gain wider acceptance among general and even medical societies.
In a quick internet search, we can find a number of clinics offering a “fetal ultrasound session for nondiagnostic purposes only,” “fetal ultrasound as a completely nondiagnostic service” or even “baby scanning providing a nonmedical souvenir.” Another issue is using pregnant women as models for training during obstetrical ultrasound courses. This does not seem to be the right direction for fetal ultrasound, which was most probably developed by those who are not completely aware of the fact that ultrasound is not just a toy and may carry potentially harmful biological effects.
■ Do we care?
As stated above, the awareness of doctors or sonographers performing fetal ultrasound is the most critical safety rule to allow us to keep the modality safe for the embryo and fetus. This assumption led the American Federal Drugs Administration to the obligation of displaying TI and MI values on the ultrasound scanner screen. However, the work will be in vain if the safety rules will not be made clear to the doctors/sonographers, or will not be followed and monitored carefully during scanning. Introducing these subjects as an obligatory part of residents’ training could potentially help. Currently, many publications prove that fetal safety is not necessarily in the range of interest of those who perform the studies [27]. The evidence of how much we, as medical professionals, care about the safety of ultrasound can be seen in a PubMed search, where among the 33,684 publications, including the expression ‘fetal ultrasound’, only 24 contained the term ‘TI’ or ‘thermal index’ and 26 the term ‘MI’ or ‘mechanical index’.
Fetal MR
There is no scientific evidence in humans to suggest that the risk to the fetus from a routine MRI examination is significantly increased during pregnancy. In 2010, the American College of Radiology has reported that present data have not conclusively documented any deleterious effects of MRI exposure on the developing fetus [28], and in 2011, they stated that there are no known adverse effects of MRI on the fetus. Moreover, MRI has been used to evaluate obstetrical, placental and fetal abnormalities in pregnant patients for more than 25 years (Figure 2), and is a proven, established imaging modality for evaluating fetal anomalies that are not well assessed with sonography [29]. MR not only contributes to diagnosis, but also serves as an important guide to treatment and delivery planning and counseling. However, fetal MRI should only be performed for a valid medical reason, and only after careful consideration of sonographic findings and family history.
Compared with fetal ultrasound, fetal MRI seems to be a relatively rarer used modality. However, only considering the Organization for Economic Co-operation and Development (OECD) countries, an average of 41.3 MRI exams are performed per 1000 population. The population of OECD countries in 2009 equaled 1,220,992,000, the number of all types of MRI scans per year for these countries was 50,000,000 MRI scans, and the number of fetal MRI scans estimated approximately at the 0.1% level, which would lead to 50,000 fetal MRI scans per year only in OECD countries (according to the European Magnetic Resonance Forum estimates from 2011). The scale increases every year together with further development of MRI diagnostics and wider acceptance and accessibility of the method.
Although there is no indication that the use of clinical MR procedures during pregnancy produces adverse effects, the positive proof of safety is difficult to achieve (International Electrotechnical Commission [IEC] 2008) and there is currently uncertainty regarding the risk associated with MRI examinations of pregnant patients, as stated by the International Commission on NonIonizing Radiation Protection 2004, 2009b, the Medicines and Healthcare products Regulatory Agency 2007 and the IEC 2008 [30]. There are also reports proving that the fetus may be more vulnerable to temperature elevations due to MRI [30,31].
Among potential adverse effects of MRI the following types may be recognized:
▪ Acoustic damage
▪ Teratogenic effects
▪ Direct nonthermal interaction of the electromagnetic field with biological structures
▪ Heating effect of MR gradient changes
▪ Risk of teratogenesis from gadolinium
Acoustic damage appears to be a theoretical rather than a real concern [32–34].
Among teratogenic effects observed in animal studies, a reduction in the crown-rump length was seen in mice exposed to MRI [35], and eye malformations in a genetically predisposed mouse strain was noticed [36]. Several hours of chick embryo exposure to a strong static magnetic field and rapid electromagnetic gradient fluctuations in the first 48 h of life resulted in an excess number of dead or abnormal chick embryos, when examined at day 5 [37]. However, recent reports show that even repetitive exposure to a 7-Telsa static magnetic field of mice in utero does not cause alterations in basal emotional and cognitive behaviour in adulthood [38].
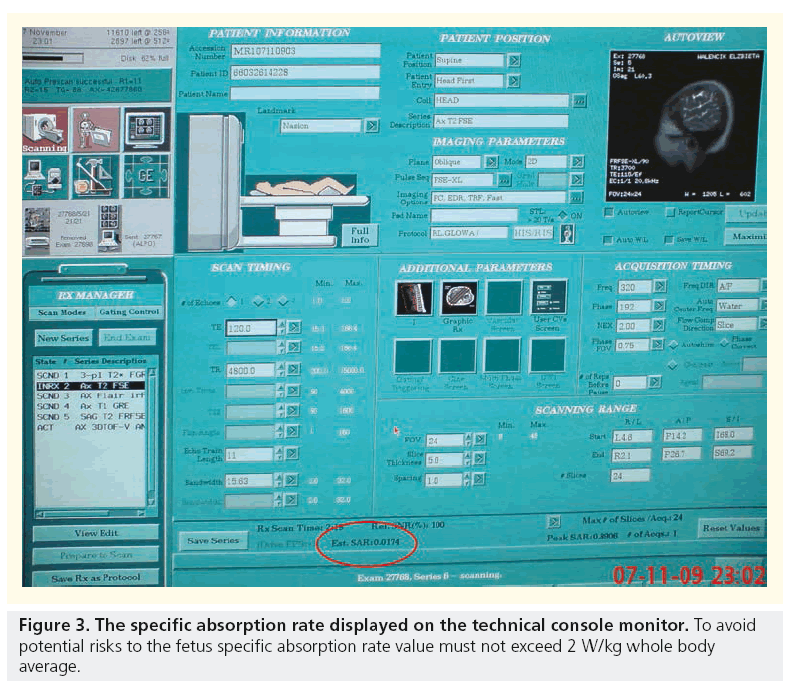
Possible mechanisms for apparent deleterious effects include direct nonthermal interaction of the electromagnetic field with biological structures and the heating effect of MR gradient changes. Direct nonthermal interaction of the electromagnetic field with biological structures does not seem to play an important role. The radiofrequency radiation pulses used in MRI, although nonionizing, result in energy deposition and can potentially lead to tissue heating. The amount of energy deposited in a patient, so called specific absorption rate (SAR), is a measure of the rate at which energy is absorbed by the body when exposed to a radio frequency electromagnetic field, defined as the power absorbed per mass of tissue, and has units of W/kg. SAR increases with static magnetic field strength, flip angle and number, and spacing of radiofrequency pulses. Therefore, single-shot echo train spin-echo sequences are associated with a relatively high SAR, while gradient-echo sequences that do not depend on radiofrequency refocusing are associated with a relatively low SAR. Despite the potential of long-echo trains to cause fetal heating, the use of single-shot echo train spin-echo sequences is common in fetal imaging and unlikely to result in significant temperature changes. Corresponding SAR values that would be necessary to cause temperature elevations in the healthy adult female are probably in the range of 15 W/kg whole body average. MRI procedures carried out under normal mode conditions, in which SAR 2 W/ kg maternal whole body is associated with fetal SAR, which is compliant with limits in exposure guidelines (ICNIRP 2004, MHRA 2007, and the IEC 2008 safety standard [39]). Moreover, tissue heating is greatest at the maternal body surface and approaches negligible levels near the body center, making it unlikely that thermal damage to the fetus is a serious risk [40].
MRI also carries the risk of teratogenesis from gadolinium. Intravenous gadolinium is teratogenic in animal studies, albeit at high and repeated doses [41]. While teratogenic effects have not been observed in a small number of human studies where gadolinium has been given in pregnancy [42,43], it is clear that gadolinium should not be administered in pregnancy, particularly during the period of organogenesis, unless there is an absolutely essential clinical indication. Gadolinium-based contrast agents pass through the placental barrier and enter the fetal circulation, they are filtered in the fetal kidneys and then excreted into the amniotic fluid in animals and humans following intravenous administration to the pregnant female. Current radiology recommendations discourage the use of gadolinium- based contrast agents during pregnancy because their safety for the fetus has not yet been proven [28]. Yet available evidence suggests it is unlikely that these compounds have an adverse effect on the developing fetus; and therefore, their use should not be limited, particularly given the important clinical reasons for MRI examinations during pregnancy.
■ How to keep it safe?
To keep fetal MRI safe, a few important rules must be kept according to the ALARA principle. First, to keep SAR under 2 W/kg whole body average, second to limit the examination time in order to avoid maternal heat stress, and third to watch fetal temperature so that it stays less than 38°C and does not rise by more than 0.5°C. Again, the awareness of doctors and sonographers about these key safety factors seems to play the most crucial role (Figure 3).
■ Do we care?
As with fetal ultrasound, a PubMed search gives some indication of how seriously medical professionals treat safety indices. Among 3687 searches including the expression ‘foetal MRI’ or ‘fetal MRI’, ‘SAR’ or ’specific absorption rate’ expression was included in 12 publications.
Do we cause fetal anomalies?
Let us try to look at the subject from the other side. Considering that many widely used imaging modalities, such as fetal ultrasound and fetal MRI could be harmful methods causing adverse effects, an increased number of fetal anomalies should be observed. It is known that teratogenic factors occurring before or during implantation of the embryo into the uterine wall do not present as subsequent developmental abnormalities. At that stage, the blastocyst is susceptible to the lethal effects, and it will either resist teratogens with no further implication or stop development. This phenomenon is called the ‘all or none’ effect [1,44]. However, teratogenic factors occurring during the period of organogenesis following implantation can result in a variety of developmental malformations, while occurrence in later stages results in reduced growth rate [1]. The next-generation effects, including postnatal growth and neurobehavioral alterations, should also be considered; however, animal experiments using various mammalian species have not been able to determine any effect on exposure levels for embryonic loss, congenital malformations and neurobehavioral effects [11,45].
European Concerted Action on Congenital Anomalies and Twins established in 1979, a European network of population-based registries for the epidemiologic surveillance of congenital anomalies, recorded a total prevalence of congenital anomalies of approximately 2% of new born babies in 27 registered countries, as published in the European Perinatal Health Report [101]. The prevalence of all anomalies per 10,000 births was 1478 in 1980, while in 1999 it increased to 16,787. This rapid increase may be explained by fast development of diagnostics during that time, which may lead to the conclusion that the number of fetal anomalies could have not significantly changed, but many more anomalies were diagnosed. More interesting is the comparison of reports from the years when fetal ultrasound and MRI where already widely spread. Comparing the reports from 1999 and 10 years later from 2009, it appears that the actual number of fetal anomalies has not only not increased, but has even slightly decreased to 14,503.
Conclusion
Despite the fact that both fetal ultrasound and MRI are generally recognized as totally safe imaging modalities for the developing fetus, there are numerous reports warning about potential harmful effects, which both modalities can carry. On the other hand, it is obvious that ultrasound and MRI are powerful diagnostic measures in pre- and peri-natal medicine. The risk–benefit ratio should always be taken into account when ultrasound and MRI are used during pregnancy. However, data published in the European Perinatal Health Reports do not show a significant increase of incidence of fetal anomalies, which may be an indirect proof that imaging modalities used for diagnostics of fetuses may not be as harmful as some publications may report. But it does not exempt medical professionals from the responsibility of gaining deeper knowledge of potential bioeffects generated by the equipment used and acquiring the practical skills of manipulating control settings that keep the imaging modalities not only consistent with achieving diagnostically useful results, but also within the range of safety.
Future perspective
Recent years show univocally that the number of diagnostic procedures, including imaging in pregnant females, is increasing constantly. This tendency will most probably continue, particularly considering the fast development in the field of medical imaging. Due to these facts, the medical society will be challenged to provide safe standards for the imaging of pregnant females, as the diagnostics of both pregnant woman and the fetus will become routine daily practice, which will need to be kept as safe as possible, while delivering highly accurate diagnostic information at the same time.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
- Ziskin MC, Morrissey J. Thermal thresholds for teratogenicity, reproduction, and development. Int. J. Hyperthermia. 27(4), 374–387 (2011).
- Salvesen KA, Eik-Nes SH. Is ultrasound unsound? A review of epidemiological studies of human exposure to ultrasound. Ultrasound Obstet. Gynecol. 6, 293–298 (1995).
- Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet 342, 887–891 (1993).
- Kieler H, Cnattingius S, Haglund B, Palmgren J, Axelsson O. Sinistrality – a sideeffect of prenatal sonography: a comparative study of young men. Epidemiology 12, 618–623 (2001).
- Salvesen KA. Ultrasound in pregnancy and non-right handedness: meta-analysis of randomized trials. Ultrasound Obstet. Gynecol. 38(3), 267–271 (2011).
- Salvesen KA, Eik-Nes SH. Ultrasound during pregnancy and subsequent childhood non-right handedness: a meta-analysis. Ultrasound Obstet. Gynecol. 13(4), 241–246 (1999).
- Campbell JD, Elford RW, Brant RF. Case-control study of prenatal ultrasonography exposure in children with delayed speech. CMAJ 149, 1435–1440 (1993).
- Sheiner E, Abramowicz JS. A symposium on obstetrical ultrasound: is all this safe for the fetus? Clin. Obstet. Gynecol. 55(1), 188–198 (2012).
- Sande RK, Matre K, Eide GE, Kiserud T. Ultrasound safety in early pregnancy: reduced energy setting does not compromise obstetric Doppler measurements. Ultrasound Obstet. Gynecol. 39(4), 438–443 (2012).
- Abramowicz JS. Foetal Doppler: how to keep it safe? Clin. Obstet. Gynecol. 53(4), 842–850 (2010).
- Jensh RP, Brent RL. Intrauterine effects of ultrasound: animal studies. Teratology 59, 240–251 (1999).
- Murai N, Hoshi K, Kang DH, Suzuki M. Effects of diagnostic ultrasound irradiated during foetal stage on emotional and cognitive behaviour in rats. Tohoku J. Exp. Med. 117, 225–235 (1975).
- Tarantal AF, Hendrickx AG. Evaluation of the bioeffects of prenatal ultrasound exposure in the cynomolgus macaque (Macaca fascicularis): II. Growth and behavior during the first year. Teratology 39, 149–162 (1989).
- Vorhees CV, Acuff-Smith KD, Schilling MA et al. Behavioral teratologic effects of prenatal exposure to continuous-wave ultrasound in unanesthetized rats. Teratology 50, 238–249 (1994).
- Norton S, Kimler BF, Cytacki EP, Rosenthal SJ. Prenatal and postnatal consequences in the brain and behavior of rats exposed to ultrasound in utero. J. Ultrasound Med. 10, 69–75 (1991).
- Scheidt PC, Stanley F, Bryla DA. One-year follow-up of infants exposed to ultrasound in utero. Am. J. Obstet. Gynecol. 131, 743–748 (1978).
- Stark CR, Orleans M, Haverkamp AD, Murphy J. Short- and long-term risks after exposure to diagnostic ultrasound in utero. Obstet. Gynecol. 63, 194–200 (1984).
- Miller MW, Church CC, Miller RK, Edwards MJ. Fetal thermal dose considerations during the obstetrician’s watch: implications for the pediatrician’s observations. Birth Defects Res. C. Embryo Today 81, 135–143 (2007).
- Edwards MJ. Review: Hyperthermia and fever during pregnancy. Birth Defects Res. A. Clin. Mol. Teratol. 76(7), 507–516 (2006).
- Miller MW, Nyborg WL, Dewey WC, Edwards MJ, Abramowicz JS, Brayman AA. Hyperthermic teratogenicity, thermal dose and diagnostic ultrasound during pregnancy: implications of new standards on tissue heating. Int. J. Hyperthermia 18(5), 361–384 (2002).
- Edwards MJ, Shiota K, Smith MS, Walsh DA. Hyperthermia and birth defects. Reprod. Toxicol. 9(5), 411–425 (1995).
- EFSUMB Study Group. CEUS Guidelines. Ultraschall Med. 29, 28–44 (2008).
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med. Biol. 17(2), 179–185 (1991).
- British Medical Ultrasound Society. Guidelines for the Safe Use of Diagnostic Ultrasound Equipment. British Medical Ultrasound Society, London, UK (2010).
- Abramowicz J, Brezinka C, Salvesen K, Ter Haar G; Bioeffects and Safety Committee; Board of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG). ISUOG statement on the non-medical use of ultrasound, 2009. Ultrasound Obstet. Gynecol. 33, 617–620 (2009).
- Bioeffects and Safety Committee, Salvesen K, Lees C, Abramowicz J, Brezinka C, Ter Haar G, Maršál K, Board of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG). ISUOG-WFUMB statement on the non-medical use of ultrasound, 2011. Ultrasound Obstet. Gynecol. 38(5), 608 (2011).
- Makikallio K, Jouppila P, Rasanen J. Human fetal cardiac function during the first trimester of pregnancy. Heart 91, 334–338 (2005).
- American College of Radiology. ACR–SPR Practice Guideline For The Safe And Optimal Performance Of Fetal Magnetic Resonance Imaging (MRI). American College of Radiology, VA, USA (2010).
- Colletti PM. Magnetic resonance procedures and pregnancy. In: Magnetic Resonance Procedures: Health Effects And Safety. Shellock FG (Ed.). CRC Press, Boca Raton, FL, USA, 149–182 (2001).
- Gowland PA, De Wilde J. Temperature increase in the foetus due to radio frequency exposure during magnetic resonance scanning. Phys. Med. Biol. 53, L15–L18 (2008).
- Miller MW, Nyborg WL, Dewey WC, Edwards MJ, Abramowicz JS, Brayman AA. Hyperthermic teratogenicity, thermal dose and diagnostic ultrasound during pregnancy: implications of new standards on tissue heating. Int. J. Hyperthermia 18, 361–384 (2002).
- Coakley FV, Glenn OA, Qayyum A, Barkovich AJ, Goldstein R, Filly RA. Fetal MRI: a developing technique for the developing patient. AJR Am. J. Roentgenol. 182, 243–252 (2004).
- Baker PN, Johnson IR, Harvey PR, Gowland PA, Mansfield P. A three-year follow-up of children imaged in utero with echo-planar magnetic resonance. Am. J. Obstet. Gynecol. 170, 32–33 (1994).
- Gover P, Hykin J, Gowland P, Wright J, Johnson I, Mansfield P. An assessment of the intrauterine sound intensity level during obstetric echo-planar magnetic resonance imaging. Br. J. Radiol. 68, 1090–1094 (1995).
- Heinrichs WL, Fong P, Flannery M et al. Midgestational exposure of pregnant balb/c mice to magnetic resonance imaging. Mag. Res. Imag. 8, 65–69 (1986).
- Tyndall DA, Sulik KK. Effects of magnetic resonance imaging on eye development in the C57BL/6J mouse. Teratology 43, 263–275 (1991).
- Yip YP, Capriotti C, Talagala SL, Yip JW. Effects of MR exposure at 1.5T on early embryonic development of the chick. J. Magn. Reson. Imaging 4, 742–748 (1994).
- Hoyer C, Vogt MA, Richter SH et al. Repetitive exposure to a 7 Tesla static magnetic field of mice in utero does not cause alterations in basal emotional and cognitive behavior in adulthood. Reprod. Toxicol. 34(1), 86–92 (2012).
- Hand JW, Li Y, Hajnal JV. Numerical study of RF exposure and the resulting temperature rise in the foetus during a magnetic resonance procedure. Phys. Med. Biol. 55(4), 913–930 (2010).
- Kanal E, Shellock FG, Talagala L. Safety considerations in MR imaging. Radiology 176, 593–606 (22) (1990).
- Okuda Y, Sagami F, Tirone P, Morisetti A, Bussi S, Masters RE. Reproductive and developmental toxicity study of gadobenate dimeglumine formulation (E7155) (3) – Study of embryo-fetal toxicity in rabbits by intravenous administration. J. Toxicol. Sci. 24(Suppl. 1), 79–87 (1999).
- Marcos HB, Semelka RC, Worawattanakul S. Normal placenta: gadolinium-enhanced dynamic MR imaging. Radiology 205, 493–496 (1997).
- Spencer JA, Tomlinson AJ, Weston MJ, Lloyd SN. Early report: comparison of breath-hold MR excretory urography, Doppler ultrasound and isotope renography in evaluation of symptomatic hydronephrosis in pregnancy. Clin. Radiol. 55, 446–453 (2000).
- Fisher JE, Acuff-Smith KD, Schilling MA et al. Behavioral effects of prenatal exposure to pulsed-wave ultrasound in unanesthetized rats. Teratology 54, 65–72 (1996).
- Fisher JE, Acuff-Smith KD, Schilling MA et al. Behavioral effects of prenatal exposure to pulsed-wave ultrasound in unanesthetized rats. Teratology 54, 65–72 (1996).
- The European Perinatal Health Report. www.europeristat.com/our-publications/ european-perinatal-health-report.html
■ Website