Special Report - Interventional Cardiology (2015) Volume 7, Issue 5
Why do we fail to achieve Glagovian atheroregression in lipid-lowering trials?
- Corresponding Author:
- Alexander N Kharlamov
De Haar Research Foundation, Rotterdam, The Netherlands, and New York, NY, USA
Tel: +31 642 666 912
E-mail: drkharlamov@icloud.com
Abstract
• Reduction of total atheroma volume provides cardiology with a hope to reverse atherosclerosis in hands of the modern-day lipidlowering medications: • ASTEROID and SATURN trials demonstrated the potential of statins which reduce the total atheroma volume up to 6.38 mm3 in coronary arteries and 30% decrease in outcomes; • Recombinant ApoA-I Milano demonstrated a 14.1 mm3 reduction in total atheroma volume with unproven effect on clinical outcomes; • ZEUS trial with ezetimibe revealed an 8.2 mm3 atheroregression. • The plaque burden with a Glagovian threshold of 40% (PAV [percent atheroma volume] above 40% at the baseline, and below 40% at the follow-up) is the ultimate criterion of the clinically valuable lesion we are able to examine in order to judge the genuine Glagovian atheroregression. • Some methodological flaws of the modern-day interventional imaging approaches including improper interpretation of the vessel contours, misunderstanding of such parameters as total atheroma volume and PAV, absence of the unified CoreLab expert methodology for assessment of both intravascular and noninvasive coronary imaging significantly impact results and further progress in this field: • There is a difference in methodology how to assess plaque burden between histology of Glagov and IVUS-imaging of Nissen; • Neither intravascular intravascular ultrasound (IVUS) or optical coherence tomography nor noninvasive computed tomography angiography allow us to comprehensively distinguish all the artery layers; • External elastic membrane and adventitia could be quantitatively assessed by IVUS only; • Some new medications as well as progress in the development of the bioresorbable scaffolds and nanomedicine promise to revolutionize the cardiovascular biomedicine with the main goal to achieve Glagovian atheroregression below 40% threshold of the PB: • The bioresorbable scaffold Absorb BVS (Abbott Vascular, CA, USA) is the first coronary device which has shown phenomena such as late lumen enlargement and wall thinning with at least 12% reduction of plaque burden; • NANOM-FIM trial of plasmonic photothermal therapy with silica-gold nanoparticles showed truly unprecedented 60.3 mm3 plaque volume reduction. • The modern-day statin trials were conducted in patients with relatively small lesions when PAV never exceeded 40% and without proper methodology, which means that the revealed phenomenon of atheroregression is essentially the pseudo reduction of the atheroma volume. • The PAV or plaque burden is the only parameter to describe changes in the vessel geometry from the Glagov phenomenon point of view because it mathematically reflects patterns between both vessel and lumen size. • IVUS with assessment of plaque burden remains the golden standard to evaluate atheroregressive patterns of medications or medical devices in clinical trials: • Trials with the baseline PAV above 40% demonstrated higher potential of the plaque burden reduction; • Further comprehensive analysis is required in order to validate the genuine threshold of the artery enlargement between a 20 and 55% PAV when positive correlation between lumen area and PB gets replaced by the negative correlation with the progressive narrowing of the lumen.
Keywords
atheroregression, bioresorbable scaffolds, coronary computed tomography angiography, Glagov phenomenon, intravascular ultrasound, lipid-lowering drugs, nanomedicine, optical coherence tomography, plaque burden, total atheroma volume
Reduction of total atheroma volume provides cardiology with a hope to reverse atherosclerosis in hands of the modern-day lipid-lowering medications
Prevention of atherosclerosis and treatment of its complications remain a clinical challenge [1]. Some recent clinical trials demonstrated [1–10] moderate atheroprotective effect of the different lipid-lowering medications. There are some achievements as well as methodological flaws, which require our attention in order to optimize the research tools for imaging and treatment in interventional cardiology with the final goal to reverse Glagov atherogenesis. HMGCoA (or 3-hydroxy-3-methyl-glutaril-CoA reductase, or HMGCR) reductase inhibitors have an outstanding track record of lowering cholesterol and improving outcomes. Clinical trials such as MIRACL (The Myocardial Ischemia Reduction with Acute Cholesterol Lowering Trial, 2001) [1], REVERSAL (The Reversal of Atherosclerosis with Lipitor, 2004) [1], PROVE IT (The Pravastatin or Atorvastatin Evaluation and Infection Therapy, 2004) [2,3], ESTABLISH (Early Statin Treatment in Patients with Acute Coronary Syndrome, 2004) [4], ASTEROID (A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden, 2006) [5], JAPAN-ACS (Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome, 2007) [7], JUPITER (The Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin, 2008) [6–9], SATURN (the Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin versus Atorvastatin, 2011) [10], and IBIS-4 (Integrated Biomarkers and Imaging Study-4) [11] have demonstrated (Table 1) that lowering LDL levels through intensive statin therapy can slow progression, or even partially reduce the total atheroma volume (TAV; up to 13.14 mm3) in coronary arteries with minimal difference in outcomes between hydrophilic and lipophilic statins. However, in these trials statin therapy was associated with only a 30% relative reduction in major cardiovascular events [1–10]. By the way of comparison, in a pilot trial recombinant ApoA-I Milano demonstrated a 14.1 mm3 reduction in total atheroma volume with unproven effect on clinical outcomes [12]. Necrotic core and calcification may limit the maximal benefit that risk factor modification and systemic drug therapy may achieve [1–10,12–21]. The most recent ZEUS (eZEtimibe Ultrasound Study, 2014) trial [14] with ezetimibe revealed a 8.2 mm3 atheroregression promising new era in the lipid-lowering management of the vulnerable patients. Some methodological flaws such as absence of the unified guidelines for imaging of coronaries, wrong interpretation of the vessel contours and adventitia, incorrect validation of plaque burden, and dramatically low percent atheroma volume at the baseline (at least below a 40% Glagovian threshold of the plaque burden) significantly impair results of the lipid-lowering trials and underestimate clinical potential of these medications.
| Clinical trial, year of publication | Study design | Intervention | Number of patients | Duration of the followup | Base line | Follow-up | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean plaque volume (TAV) | Mean plaque burden (PAV) | Mean absolute change in plaque volume (TAV)* | Mean relative change in plaque volume (TAV)* | Mean change in plaque burden (PAV)* | |||||
| Statin drugs | |||||||||
| REVERSAL, 2004 [1] | RCT double blind | Atorvastatin 80 mg (vs pravastatin 40 mg) | 253 (vs 249) | 18 months | 184.4 mm3 | 38.4% | -0.9 mm3 (median) -0.4 mm3 [mean] | -0.4% (vs +2.7%) | +0.2% (median) +0.6% [mean] |
| CAMELOT, 2004 [2] | RCT placebo controlled | Amlodipine 10 mg (vs enalapril 20 mg and placebo) | 663 (vs 673 and 655) | 24 months | NA | 39.9% | NA | NA | +0.5% (vs +0.8% and +1.3%) |
| A-Plus, 2004 [3] | RCT double blind placebo controlled | Avasimibe 750 mg | 117 | 24 months | 202.3 mm3 | 45.3% | +1.9% (NS) | +1.0% (NS) | +1.0% (NS) |
| ESTABLISH, 2004 [4] | RCT | Atorvastatin 20 mg daily | 24 | 6 months | 69.6 mm3 | 29.9% | -8.3 mm3 | -13.1% | -3.8% |
| ASTEROID, 2006 [5] | Prospective open-label blinded end points | Rosuvastatin 40 mg daily | 349 | 24 months | 212.2 mm3 | 39.6% | -12.5 mm3 (median) -14.7 mm3 [mean] | -2.9% | -0.79% (median) -0.98% [mean] |
| ACTIVATE, 2006 [6] | RCT placebo controlled | Pactimibe 100 mg daily (ACAT inhibitor) | 554 | 18 months | 198.1 mm3 | 39.8% | -1.3 mm3 (vs -5.6 mm3 in placebo) | -0.7% | +0.69% |
| PERISCOPE, 2008 [6] | RCT double blind | Pioglitazone 15 to 45 mg (vs glimepiride 1 to 4 mg) | 543 | 18 months | NA | 40.6% | NA | NA | -0.16% (vs +0.73%) |
| ILLUSTRATE, 2008 [6] | RCT | Torcetrapib/ atorvastatin | 910 | 24 months | NA | 37.0% | NA | NA | +0.1% (NS) |
| STRADIVARIUS, 2008 [6] | RCT placebo controlled | Rimonabant (anti-obesity drug) | 839 | 20 months | 191.7 mm3 | 37.5% | -2.2 mm3 | -1.04% | +0.25% |
| JAPAN-ACS, 2009 [7] | RCT openlabel parallel | Atorvastatin 20 mg daily (vs pitavastatin 4 mg daily) | 252 | 12 months | 63.9 mm3 | 50.5% | -10.6 mm3 (vs -8.2 mm3) | -18.1% (vs -16.9%) | -6.3% (vs -5.7%) |
| COSMOS, 2009 [8] | Open-label observational | Rosuvastatin 2.5 mg daily with titration up to 20 mg daily | 214 | 15 months | NA | NA | NA | -5.1% | NA |
| TOGETHAR, 2010 [9] | Open-label observational | Pitavastatin 2 mg daily | 90 | 12 months | NA | NA | NA | NS | NA |
| SATURN, 2011 [10] | RCT double blind | Rosuvastatin 40 mg daily (vs atorvastatin 80 mg) | 520 (vs 519) | 24 months | 144.1 mm3 | 36.7% | -6.39 mm3 (vs -4.42 mm3) (median) -8.4 mm3 (vs -5.7 mm3) (mean) | -5.8% (vs -3.9%) | -1.22% (vs -0.99%) (median) -1.3% (vs -1.1%) (mean) |
| IBIS-4, 2015 [11] | Prospective cohort study | Rosuvastatin 40 mg daily | 103 | 13 months | 258.3 mm3 | 43.9% | -13.14 mm3 | -5.09% | -0.90% (median) |
| Lipid-lowering medications | |||||||||
| ApoAI-Milano, 2003 [11] | RCT double blind placebo controlled | Combined ETC- 216 15 mg/kg and 45 mg/kg (five weekly infusions) | 36 | 5 weeks | 268.4 mm3 | 38.96% | -13.3 mm3 (median) -14.1 mm3 [mean] | -5.2% | -1.06% (median) -0.81% [mean] |
| IBIS-2, 2008 [12] | RCT double blind placebo controlled | Darapladib 160 mg daily orally (LpPLA2 inhibitor) | 175 | 12 months | 327 mm3 | 40.7% | -5.0 mm3 | -0.9% | NS |
| ZEUS, 2014 [13] | Prospective study | Ezetimibe 10 mg/day + atorvastatin 20 mg/day vs atorvastatin 20 mg/day | 50 (vs 45) | 6 months | 75.1 mm3 (vs 76.5 mm3) | 47.5% (vs 46.7%) | -8.2 mm3 (vs -6.2 mm3) | NA | -12.5% (vs -7.5%) |
| PRECISE-IVUS, 2014# | RCT openlabel | Ezetimibe 10 mg/dl + Atorvastatin vs atorvastatin | 245 | 12 months | NA | NA | NA | NA | NA |
| OUHOCTIVUS, 2014# | RCT double blind placebo controlled | Ezetimibe 10 mg/dl + 80 mg Atorvastatin vs 80 mg Atorvastatin | 87 | 12 months | NA | NA | NA | NA | NA |
| GLAGOV (Phase III), 2016# | RCT double blind placebo controlled | Evolocumab/ AMG 145 (PCSK9 MAb) | 970 | 20 months | NA | NA | NA | NA | NA |
| Animal studies of mTOR inhibitors, 2002–2013 [14] | NA | Rapamycin 0.01–8 mg/ kg/day (mTOR inhibitor) | NA | 1–3 months | NA | NA | NA | NA | -0.4–85% |
| Coronary devices | |||||||||
| ABSORB A, 2009 [16] | Prospective open-label study | Implantation of the bioresorbable scaffold Absorb BVS (Abbott Vascular, CA, USA) | 29 | 6-24 months | 116.9 mm3 (6 months postprocedure) | 62.3% | -13.38 mm3 (median) -18.24 mm3 (mean) | -15.6% (from 6 months) | -6.9% |
| PLASMONICS, 2008 [17] | Bench study, Yukatan swines on western diet | MICS implantation onto coronary artery of the bioengineered patch bearing gold nanoparticles with further intravascular transcatheter PPTT by nearinfrared laser | 101 | 12 months | 179.6 mm3 | 60.9% | -79.4 mm3 | -44.2% | -29.8% |
| NANOM-FIM, 2012 [18] | Prospective observational study | The same as above in PLASMONICS study | 60 | 12 months | 178.4 mm3 | 68.5% | -60.3 mm3 | -33.8% | -30.7% |
TAV and PAV calculated by analysis of IVUS.
ACAT: The enzyme acyl-CoA : cholesterol acyltransferase; BVS: Bioresorbable vascular scaffold; IVUS: Intravascular ultrasound; LpPLA2: Lipoprotein-associated phospholipase A2; MICS: Minimally invasive cardiac surgery; NA: Non-available or Not applicable; NS: Non-significant changes of variables (p-value > 0.05); PAV: Percent atheroma volume; PCSK9: Proprotein convertase subtilisin kexin 9; PPTT: Plasmonic photothermal therapy; RCT: Randomized controlled trial; TAV: Total atheroma volume (mm3).
Table 1. Glagov atheroregression in the trials with the drug treatment and implantation of coronary devices.
The Glagov atheroregression below a 40% threshold of plaque burden as the ultimate goal of atheroprotective strategy
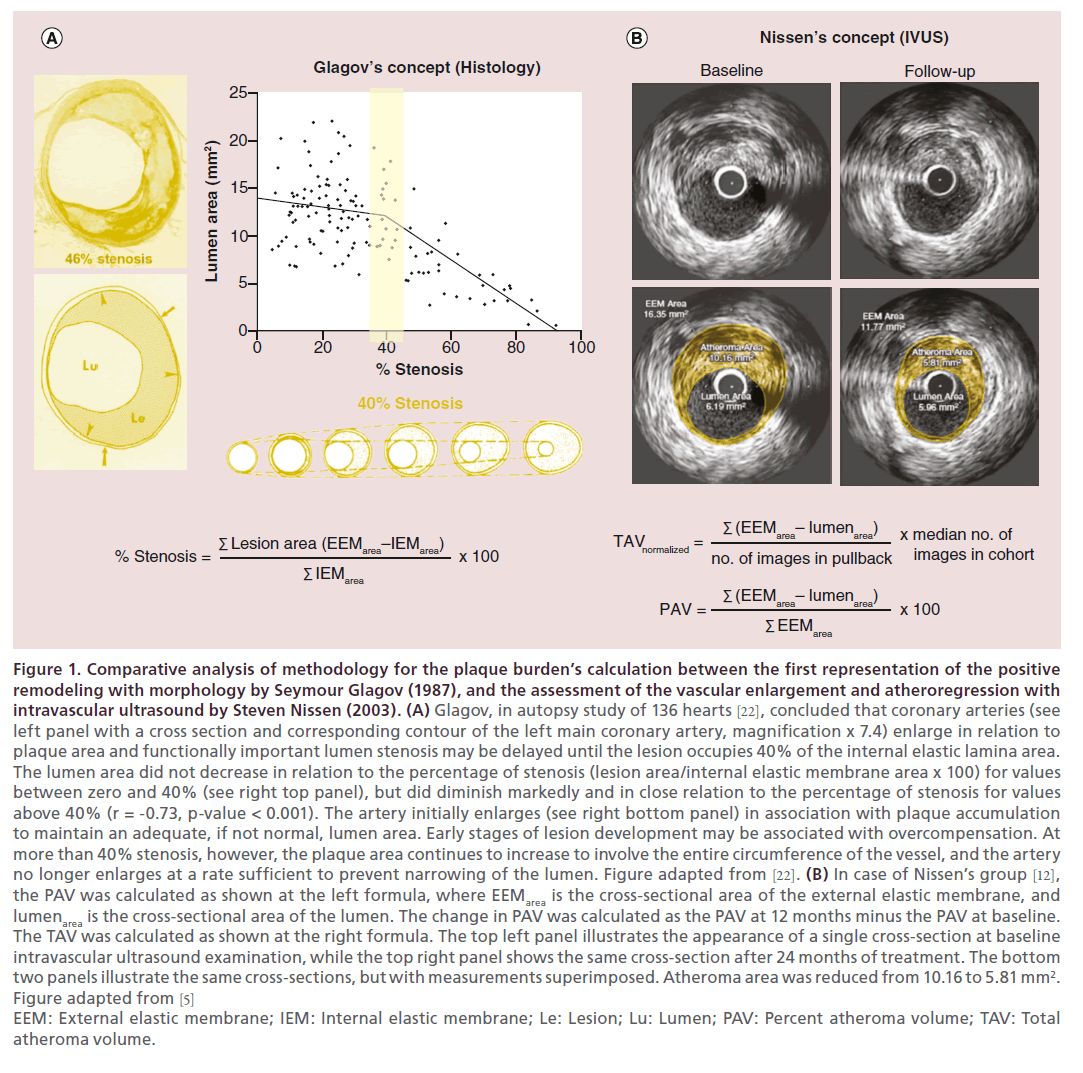
New generations of devices may help fulfill the ultimate goal of atheroregression below the Glagov threshold by reversing atherogenesis, slowing aging and triggering repair of diseased arteries. The Glagov’s observation [22] in 1987 (see Figure 1) suggests that vascular remodeling maintains the artery lumen dimensions as long as the plaque burden (PB) threshold of 40% is not surpassed, representing the limit where the growth of the plaque can no longer be accommodated by external elastic membrane (EEM) expansion. This process of EEM enlargement in accommodating the plaque and maintaining the lumen dimensions is referred to as the Glagov phenomenon, which is a cornerstone concept in atheroprotective strategies. Although Glagov phenomenon was originally described only for the case of arterial remodeling in response to growth of atherosclerotic plaques, experimental and clinical observations indicate that blood flow properties influence remodeling after angioplasty, hypertension and flow diversion as well as atherosclerotic plaque progression with the target 40–55% threshold of the PB [23]. The PROSPECT study [24] documented significant positive correlation between cross-sectional areas of EEM, plaque and media when PB was below the 20% threshold. That strength of the positive correlation was lost when PB increased beyond 40% due to functional exhaustion of the compensatory vessel enlargement ultimately resulting in luminal narrowing indicated by a negative significant correlation between lumen area and PB.
Figure 1. Comparative analysis of methodology for the plaque burden’s calculation between the first representation of the positive remodeling with morphology by Seymour Glagov (1987), and the assessment of the vascular enlargement and atheroregression with intravascular ultrasound by Steven Nissen (2003). (A) Glagov, in autopsy study of 136 hearts [22], concluded that coronary arteries (see left panel with a cross section and corresponding contour of the left main coronary artery, magnification x 7.4) enlarge in relation to plaque area and functionally important lumen stenosis may be delayed until the lesion occupies 40% of the internal elastic lamina area. The lumen area did not decrease in relation to the percentage of stenosis (lesion area/internal elastic membrane area x 100) for values between zero and 40% (see right top panel), but did diminish markedly and in close relation to the percentage of stenosis for values above 40% (r = -0.73, p-value < 0.001). The artery initially enlarges (see right bottom panel) in association with plaque accumulation to maintain an adequate, if not normal, lumen area. Early stages of lesion development may be associated with overcompensation. At more than 40% stenosis, however, the plaque area continues to increase to involve the entire circumference of the vessel, and the artery no longer enlarges at a rate sufficient to prevent narrowing of the lumen. Figure adapted from [22]. (B) In case of Nissen’s group [12], the PAV was calculated as shown at the left formula, where EEMarea is the cross-sectional area of the external elastic membrane, and lumenarea is the cross-sectional area of the lumen. The change in PAV was calculated as the PAV at 12 months minus the PAV at baseline. The TAV was calculated as shown at the right formula. The top left panel illustrates the appearance of a single cross-section at baseline intravascular ultrasound examination, while the top right panel shows the same cross-section after 24 months of treatment. The bottom two panels illustrate the same cross-sections, but with measurements superimposed. Atheroma area was reduced from 10.16 to 5.81 mm2. Figure adapted from [5] EEM: External elastic membrane; IEM: Internal elastic membrane; Le: Lesion; Lu: Lumen; PAV: Percent atheroma volume; TAV: Total atheroma volume.
Which imaging modalities in interventional cardiology allow us to assess plaque burden?
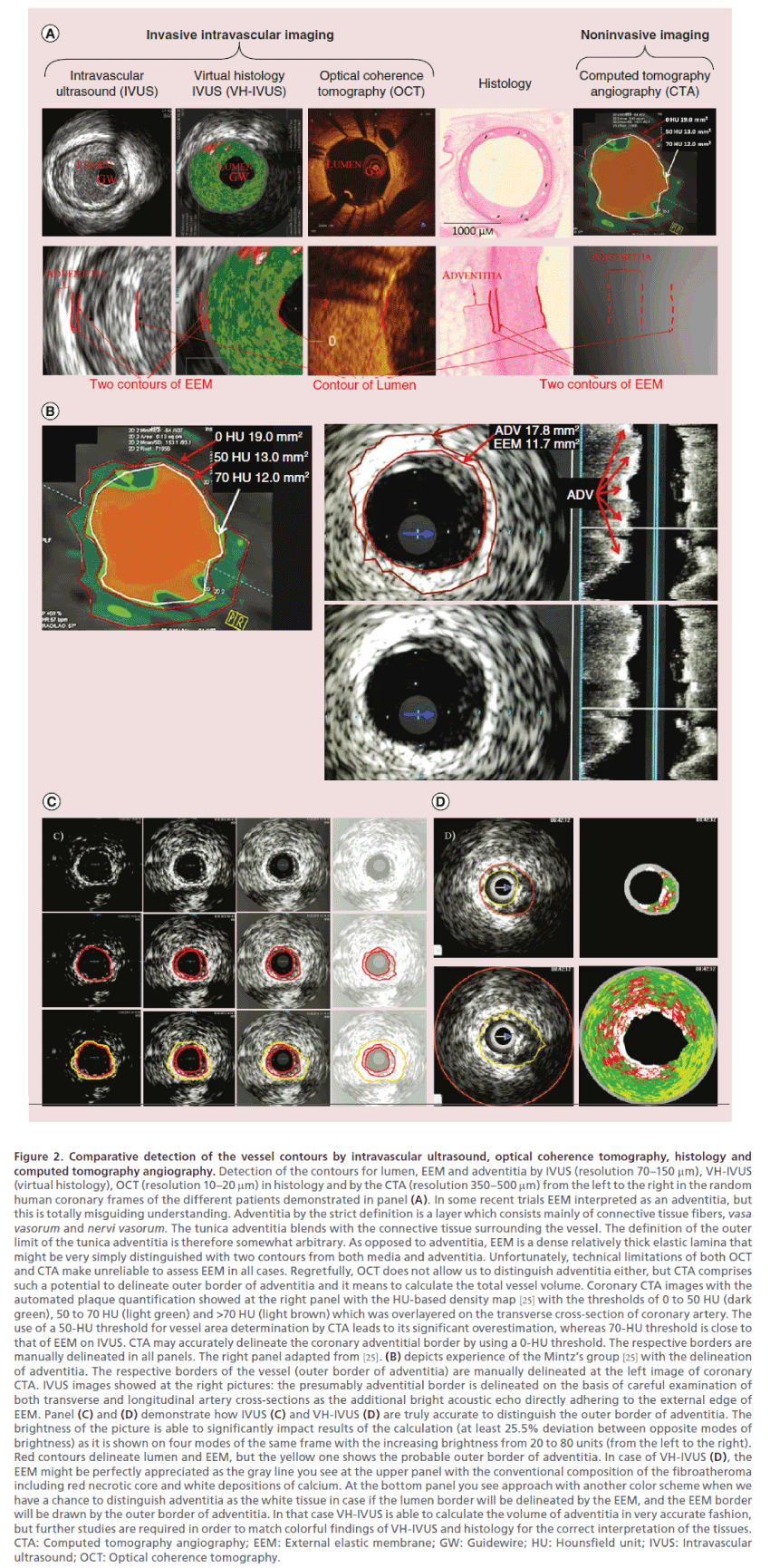
Definitely, there is a gap between both Glagov’s and Nissen’s approach to assess the dimensions of the vessel wall and it means to correctly clarify what is a threshold of the artery wall enlargement (Figure 1). Glagov could calculate the genuine size of the lesion at his morphologic samples between EEM and internal elastic membrane, nothing to talk about thickness of adventitia. The intravascular ultrasound (IVUS) with approach of the Nissen’s group [5] granted us with a tool to detect and measure lesion in alive patient but with another resolution and accuracy strictly between visible EEM and lumen, which means we are able to assess so called plaque-media size only. Neither IVUS or optical coherence tomography (OCT) nor computed tomography angiography (CTA) allow us to comprehensively distinguish all the artery layers (Figure 2). Moreover, the unified approach for the CoreLab three-head expert analysis is required The modern trials commit a sin by the excessively free interpretation of the vessel contours, which is able to significantly impact results (at least 13.1% deviation by the data of NANOM-FIM trial [19] in case of the EEM wrong detection, p < 0.05) [26]. The plaquemedia volume must be calculated strictly between internal contour of EEM and lumen. Both EEM and adventitia are mostly invisible in OCT images, meanwhile, CTA delineates just the approximate outer border of adventitia, which let us to judge total vessel volume (TVV) only (Figure 2). The current modernday IVUS approach to define TVV [17] is essentially incorrect because it comprises exclusively the external contour of EEM, but not adventitia. The correct definition of adventitia must be formulated [26] for interventional cardiology. Regretfully, we failed to propose a methodology how to measure adventitia and particularly its external contour by both IVUS and histology. The adventitia is essentially a layer without clear outer border if compared with the dense twocircuit EEM, which is sometimes wrongly interpreted as an adventitia by interventionists. Moreover the size of adventitia substantially varies in the different stage lesions, but there are no studies with the special focus on adventitia remodeling [27] within the concept of the natural history of atherogenesis which means CTA-related TVV is not that informative to track progress of atherosclerosis. The experience of the Mintz’s group [25] is perfect in sense of the methodology to match IVUS and CTA images (Figure 2), but the accuracy of IVUS remains debatable especially in sense of the quest for the optimal approach to calculate the volume of adventitia. Further studies matching IVUS, virtual histology-IVUS and histology are able to upgrade our understanding of both how to delineate the vessel contours and what is the natural history of artery remodeling. Regretfully, there is no way to assess the true PB by 64- or 128-slice CTA due to absence of the tools to verify EEM for today. In case of the quantitative coronary angiography (QCA) we can judge PB only obliquely by the percent of stenosis minding the fact that narrowing of the lumen becomes possible only in case if PB stays above 40%. These factors can partly explain why we do not see the genuine Glagov atheroregression in case of the modern-day lipid-lowering trials.
Figure 2. Comparative detection of the vessel contours by intravascular ultrasound, optical coherence tomography, histology and computed tomography angiography. Detection of the contours for lumen, EEM and adventitia by IVUS (resolution 70–150 μm), VH-IVUS (virtual histology), OCT (resolution 10–20 μm) in histology and by the CTA (resolution 350–500 μm) from the left to the right in the random human coronary frames of the different patients demonstrated in panel (A). In some recent trials EEM interpreted as an adventitia, but this is totally misguiding understanding. Adventitia by the strict definition is a layer which consists mainly of connective tissue fibers, vasa vasorum and nervi vasorum. The tunica adventitia blends with the connective tissue surrounding the vessel. The definition of the outer limit of the tunica adventitia is therefore somewhat arbitrary. As opposed to adventitia, EEM is a dense relatively thick elastic lamina that might be very simply distinguished with two contours from both media and adventitia. Unfortunately, technical limitations of both OCT and CTA make unreliable to assess EEM in all cases. Regretfully, OCT does not allow us to distinguish adventitia either, but CTA comprises such a potential to delineate outer border of adventitia and it means to calculate the total vessel volume. Coronary CTA images with the automated plaque quantification showed at the right panel with the HU-based density map [25] with the thresholds of 0 to 50 HU (dark green), 50 to 70 HU (light green) and >70 HU (light brown) which was overlayered on the transverse cross-section of coronary artery. The use of a 50-HU threshold for vessel area determination by CTA leads to its significant overestimation, whereas 70-HU threshold is close to that of EEM on IVUS. CTA may accurately delineate the coronary adventitial border by using a 0-HU threshold. The respective borders are manually delineated in all panels. The right panel adapted from [25]. (B) depicts experience of the Mintz’s group [25] with the delineation of adventitia. The respective borders of the vessel (outer border of adventitia) are manually delineated at the left image of coronary CTA. IVUS images showed at the right pictures: the presumably adventitial border is delineated on the basis of careful examination of both transverse and longitudinal artery cross-sections as the additional bright acoustic echo directly adhering to the external edge of EEM. Panel (C) and (D) demonstrate how IVUS (C) and VH-IVUS (D) are truly accurate to distinguish the outer border of adventitia. The brightness of the picture is able to significantly impact results of the calculation (at least 25.5% deviation between opposite modes of brightness) as it is shown on four modes of the same frame with the increasing brightness from 20 to 80 units (from the left to the right). Red contours delineate lumen and EEM, but the yellow one shows the probable outer border of adventitia. In case of VH-IVUS (D), the EEM might be perfectly appreciated as the gray line you see at the upper panel with the conventional composition of the fibroatheroma including red necrotic core and white depositions of calcium. At the bottom panel you see approach with another color scheme when we have a chance to distinguish adventitia as the white tissue in case if the lumen border will be delineated by the EEM, and the EEM border will be drawn by the outer border of adventitia. In that case VH-IVUS is able to calculate the volume of adventitia in very accurate fashion, but further studies are required in order to match colorful findings of VH-IVUS and histology for the correct interpretation of the tissues. CTA: Computed tomography angiography; EEM: External elastic membrane; GW: Guidewire; HU: Hounsfield unit; IVUS: Intravascular ultrasound; OCT: Optical coherence tomography.
Transient scaffolding of coronaries & nanotechnologies promise new revolution in theranostics of atherosclerosis
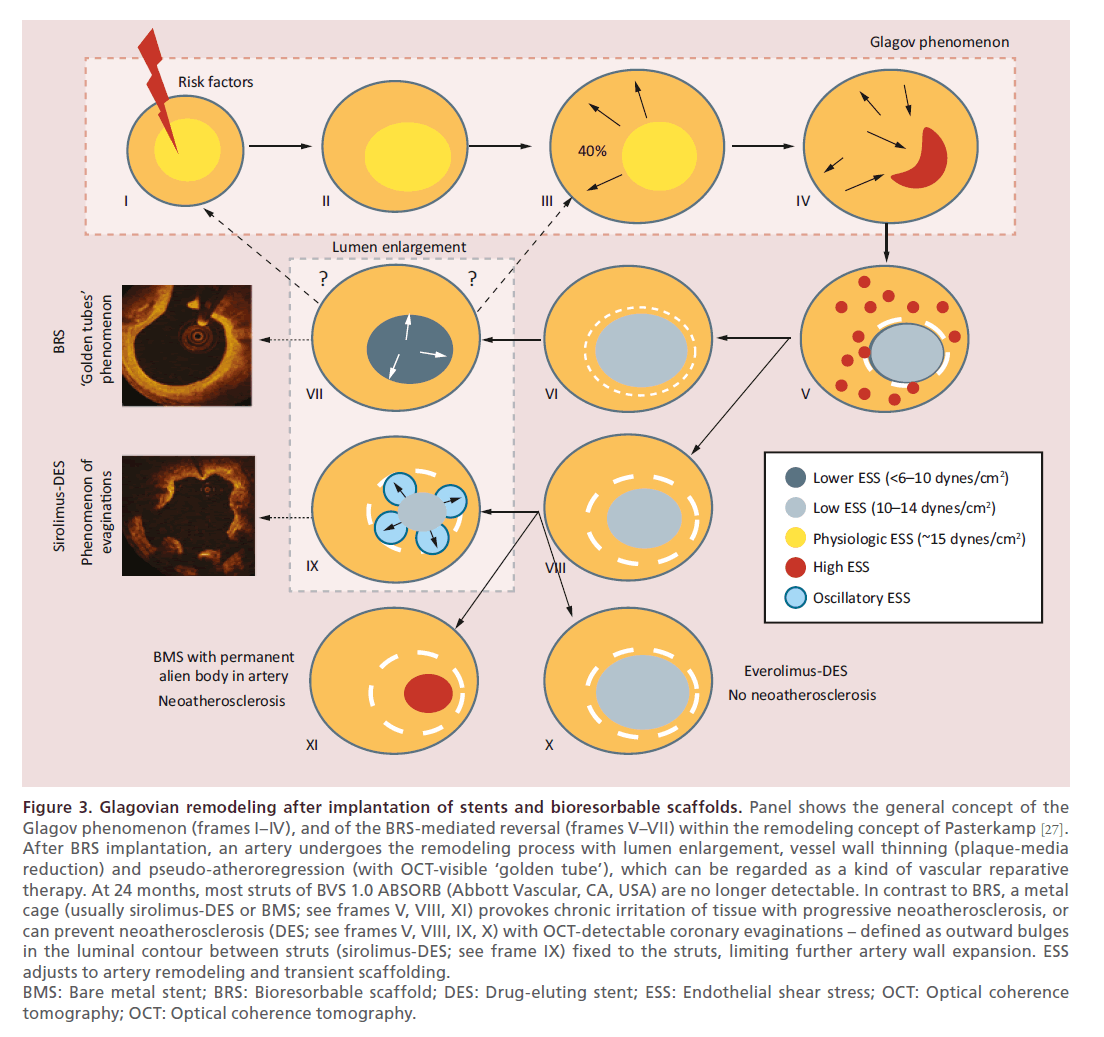
To date, the bioresorbable scaffold Absorb BVS (Abbott Vascular, CA, USA) is the first coronary device which has shown phenomena such as late lumen enlargement (without pathological remodeling) and Glagovian wall thinning with at least 12% reduction of PB [16,17] (see Table 1 & Figure 3). Bioresorbable scaffold may represent a new era in cardiovascular medicine, since interventions will address not only the obstructive component of atherosclerotic disease, but also the biologic and functional properties of the vessel. In fact, Absorb BVS in combination with other state-of-the-art approaches has a potential to pave the way for a new era of atheroregression and so-called by the team of Serruys PW [13,16–17], vascular reparative therapy. For today, the extensive experience in over 100 thousand patients demonstrates such advantages of BRS as reduction of late events (ABSORB EXTEND, 2014), restored vessel function (ABSORB cohort B trial, 2014), reduced revascularization rates (ABSORB II, 2014), plaque regression (a multi-imaging modality study in 2014 documented a biphasic change of the total plaque area shows a biphasic change with an increase between the first and second year and a plaque reduction between the second and third-year follow-up) [28], and lumen gain (ABSORB cohort B trial, 2011, 2013). Definitely, BRS performs well in STEMI patients if compare with DES, but thrombosis raises concerns. Running ABSORB III and IV trials aim to prove superiority of BRS [16,29].
Figure 3. Glagovian remodeling after implantation of stents and bioresorbable scaffolds. Panel shows the general concept of the
Glagov phenomenon (frames I–IV), and of the BRS-mediated reversal (frames V–VII) within the remodeling concept of Pasterkamp [27].
After BRS implantation, an artery undergoes the remodeling process with lumen enlargement, vessel wall thinning (plaque-media
reduction) and pseudo-atheroregression (with OCT-visible ‘golden tube’), which can be regarded as a kind of vascular reparative
therapy. At 24 months, most struts of BVS 1.0 ABSORB (Abbott Vascular, CA, USA) are no longer detectable. In contrast to BRS, a metal
cage (usually sirolimus-DES or BMS; see frames V, VIII, XI) provokes chronic irritation of tissue with progressive neoatherosclerosis, or
can prevent neoatherosclerosis (DES; see frames V, VIII, IX, X) with OCT-detectable coronary evaginations – defined as outward bulges
in the luminal contour between struts (sirolimus-DES; see frame IX) fixed to the struts, limiting further artery wall expansion. ESS
adjusts to artery remodeling and transient scaffolding.
BMS: Bare metal stent; BRS: Bioresorbable scaffold; DES: Drug-eluting stent; ESS: Endothelial shear stress; OCT: Optical coherence
tomography; OCT: Optical coherence tomography.
At present, although atheroregression below the Glagovian threshold has not been yet achieved, developments in nanotechnologies may ultimately realize this goal. A single multifunctional gold nanoparticle-based platform (‘mix-and-match’ with suitably selected components for each individual application) incorporating multiple receptor targeting, multimodality imaging (ex vivo and in vivo) and multiple therapeutic entities (molecular target therapy, atheroregression and thrombolysis) in a close interaction with near-infrared laser technologies may provide the ultimate ‘magic gold bullet’ for interventional vascular medicine [20,21].
Plasmonics, and particularly plasmonic photothermal therapy is a novel and promising approach that can be combined with metal nanoparticles. When nanoparticles are irradiated with a near-infrared laser, they absorb energy, which is quickly transferred through the nonradiative relaxation into heat which leads to irreparable damage of tissue. Systematic experimental (2001–2010) [18] and human (2007–2010) [19] studies over the last 15 years have demonstrated the main pros and cons of plasmonic photothermal therapy with the different delivery approaches. In bench tests (PLASMONICS study) [18], the mean PB reduction achieved 79.4 mm3 with the use of mini-invasive surgery-based implantation of bioengineered patches on the artery with fixation to the myocardium. The NANOM-FIM trial [19] showed truly unprecedented 60.3 mm3 plaque volume reduction at 12 months in 60 patients using similar nanotechnology (see Table 1). The concept of this approach was validated by another group [21], but these results require further investigation and need to be confirmed in larger studies.
What we expect from the small lesions in the lipid-lowering trials?
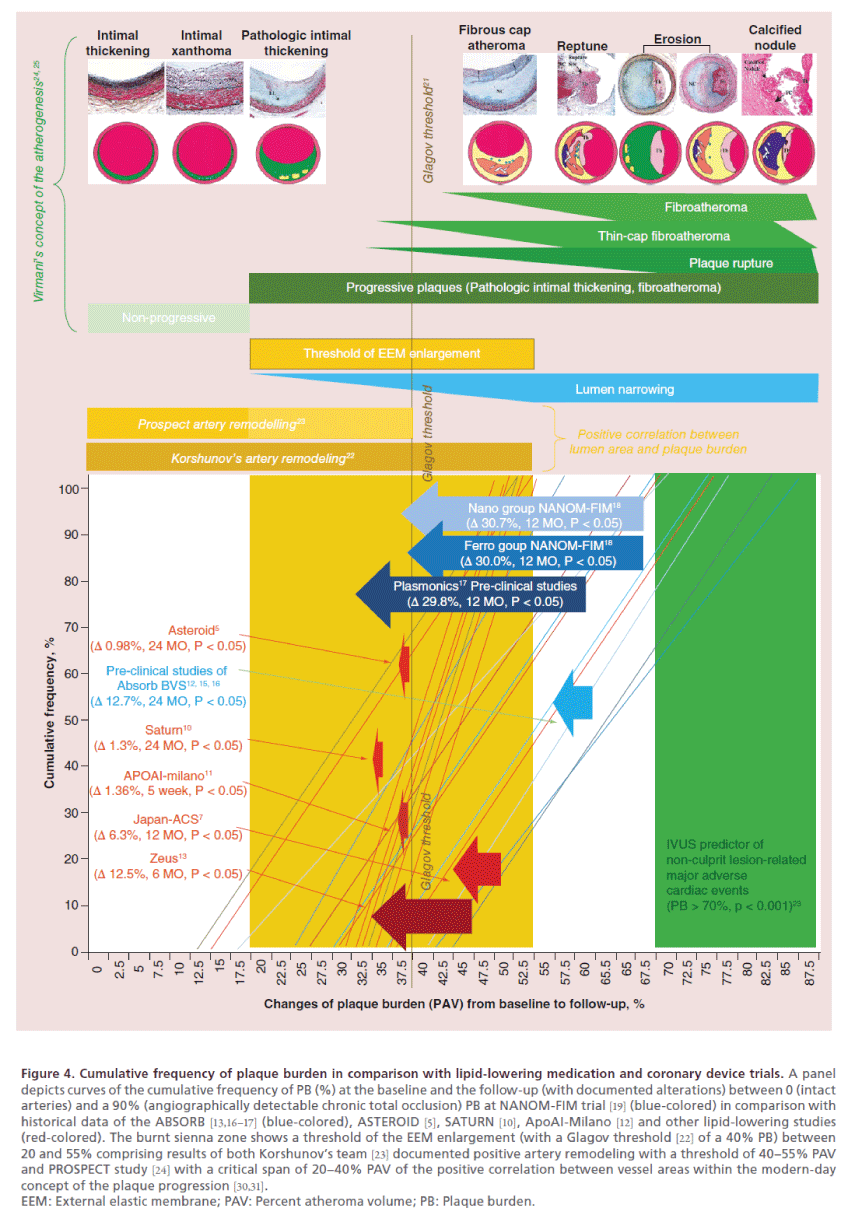
The findings of NANOM-FIM trial [19] and historical results of statin studies are not entirely comparable due to significant difference between populations and baseline parameters such as TAV, TVV and PB at the compensated vessels without signs of the true positive or negative remodeling. In most statin trials (Figure 4), the baseline PB was below 40% which means already beyond Glagov threshold. These lesions cannot be interpreted as fibroatheromas, and, moreover, PROSPECT study [24] affirmed a 70% PB as the independent predictor of the major cardiac adverse events in non-culprit lesions, which means we cannot expect any major outcomes in those trials either. In that case here is a question what we try to achieve in such young and most probably clinically silent lesions. On the one hand, plaques at those trials could be characterized as relatively small and early-stage lesions that a priori makes them more sensitive to the intensive drug therapy in comparison with the late-stage and advanced fibroatheromas with pronounced inorganic component. On the other hand, these tiny lesions depreciate the atheroprotective potential of the drug agent or device due to low initial vessel volume, which means the clinical value of the approach could be merely underestimated.
Figure 4. Cumulative frequency of plaque burden in comparison with lipid-lowering medication and coronary device trials. A panel
depicts curves of the cumulative frequency of PB (%) at the baseline and the follow-up (with documented alterations) between 0 (intact
arteries) and a 90% (angiographically detectable chronic total occlusion) PB at NANOM-FIM trial [19] (blue-colored) in comparison with
historical data of the ABSORB [13,16–17] (blue-colored), ASTEROID [5], SATURN [10], ApoAI-Milano [12] and other lipid-lowering studies
(red-colored). The burnt sienna zone shows a threshold of the EEM enlargement (with a Glagov threshold [22] of a 40% PB) between
20 and 55% comprising results of both Korshunov’s team [23] documented positive artery remodeling with a threshold of 40–55% PAV
and PROSPECT study [24] with a critical span of 20–40% PAV of the positive correlation between vessel areas within the modern-day
concept of the plaque progression [30,31].
EEM: External elastic membrane; PAV: Percent atheroma volume; PB: Plaque burden.
Total atheroma volume or percent atheroma volume: what variable is more favorable to judge atheroregression?
Moreover, the TAV remains a kind of the ‘gold standard’ to assess real absolute alterations in the atheromatous lesions being meanwhile essentially less informative if compared with the percent atheroma volume (PAV). The PAV is the only parameter to describe changes in the vessel geometry from the Glagov phenomenon point of view because it mathematically reflects patterns between both vessel and lumen size. In case of TAV we ignore sometimes the size of the artery wall and natural history of the vessel remodeling which means our achievements might be misinterpreted. The best example of such misguidance is two Chinese meta-analyses with a focus on the effects of the statin therapy on regression of coronary atherosclerosis using IVUS. The group of Gao WQ [32] reported meta-analysis of 20 trials with 5910 patients, and concluded that intensive lowering LDL-cholesterol (LDL-C) (rosuvastatin mean 33 mg daily and atorvastatin mean 60 mg daily) therapy with a >17-month duration could lead to the regression of the lesions (TAV -0.162 mm3, p = 0.0001; -0.101 mm3, p = 0.016; respectively). Moreover, LDL-C level should be reduced by >40% or to a target level of <78 mg/dl. Another analysis (17 trials, 2171 patients) of Tian J [33] documented that statin therapy (especially that involving a high dose and long duration and achieving <100 mg/dl LDL-C levels) can significantly decrease TAV (-5.3 mm3; 95% CI: -3.3 mm3 to -7.2 mm3; p < 0.001). Unfortunately, in both cases dynamics of more informative parameter – a PB (PAV) – was not taken into account. Furthermore, one of the first CTA trials of statins [34] documented a 47.7 mm3 TAV regression of the noncalcified plaques. Regretfully, authors technically measured not even TAV, but TVV with adventitia. In that case we cannot judge atheroregression just because the remodeling of adventitia substantially affects results. There in the automated quantified lesions with a low (>30%) Hounsfield unit threshold neither lumen size nor PAV were provided, which makes this trial overestimated and pointless.
How to optimize the strategy to examine atheroprotective agents?
In fact, in order to optimize our results we are obliged to pay more attention to the different imaging modalities and proceed with the IVUS or a 70 Hounsfield unit CTA assessment of PB at any trial, which has an objective to estimate Glagovian atheroregression. Potentially, neither vessel size or TAV nor narrowing of the lumen per se is of intrinsic importance. The only point there is the PB with a threshold of 40% (PAV above 40% at the baseline, and below 40% at the follow-up) as the ultimate criterion of the clinically valuable lesion we are able to examine in order to judge the real atheroregression. We know that all the trials with the baseline PAV above 40% demonstrated higher atheroregressive potential (for instance, ASTEROID [5] vs JAPAN-ACS [7] with the baseline PAV of 39.6 vs 50.5% and further reduction of PB up to 0.98 vs 6.3% respectively, p < 0.05) due to most probably activated Glagovian mechanisms of the artery remodeling and higher sensitivity to any intrinsic or extrinsic factors. Further comprehensive analysis is required in order to validate the genuine threshold of the artery enlargement between a 20 and 55% PAV when positive correlation between lumen area and PB get replaced by the negative correlation with the progressive narrowing of the lumen. Furthermore, the correlation between initial PAV in intact coronaries and degree of atheroregression as well as clinical outcomes at the follow-up must be clarified either.
Future perspective
Two aggressive statin therapy trials SATURN and IBIS-4 demonstrated very controversial results with atheroregression up to 1.22% [35], reduction of fibrous tissue and certain amount of intramural lipids, but with very slight effect on necrotic core accelerating calcium deposition whereas the fact that numerous studies of the cholesterol lowering strategies have failed to document a mortality benefit, and, so, the benefits of statins may have been overstated [36,37] which require further validation. So, the adoption of transient scaffolding with bioresorbable platforms and progress of nanomedicine in hands of the harmonized imaging have become the most compelling breakthroughs of theranostics in interventional cardiology, offering potential solutions in the imaging, targeting and treatment of atherosclerosis with the ultimate goal to achieve atheroregression below 40% Glagov threshold.
Acknowledgements
We appreciate intellectual contribution to the development of the intravascular and noninvasive imaging by the CoreLab experts of Cardialysis BV and Erasmus MC under research mentorship of PW Serruys (Rotterdam, the Netherlands) as well as software developments of Pie Medical (Maastricht, the Netherlands) and Medis Medical Imaging Systems (Leiden, The Netherlands), which inspired us to write this article. The access to NANOM-FIM trial [19] was provided by J Gabinsky (Ural Institute of Cardiology, Yekaterinburg, Russia) which was essential for the proper interpretation of the artery remodeling data. Some original pictures originated from the Department of Interventional Cardiology of Erasmus University Rotterdam Medical Center (Erasmus MC, Rotterdam, the Netherlands) and granted by the individual research fellowship project in interventional cardiology in 2012.
Executive summary
• Reduction of total atheroma volume provides cardiology with a hope to reverse atherosclerosis in hands of the modern-day lipidlowering medications:
• ASTEROID and SATURN trials demonstrated the potential of statins which reduce the total atheroma volume up to 6.38 mm3 in coronary arteries and 30% decrease in outcomes;
• Recombinant ApoA-I Milano demonstrated a 14.1 mm3 reduction in total atheroma volume with unproven effect on clinical outcomes;
• ZEUS trial with ezetimibe revealed an 8.2 mm3 atheroregression.
• The plaque burden with a Glagovian threshold of 40% (PAV [percent atheroma volume] above 40% at the baseline, and below 40% at the follow-up) is the ultimate criterion of the clinically valuable lesion we are able to examine in order to judge the genuine Glagovian atheroregression.
• Some methodological flaws of the modern-day interventional imaging approaches including improper interpretation of the vessel contours, misunderstanding of such parameters as total atheroma volume and PAV, absence of the unified CoreLab expert methodology for assessment of both intravascular and noninvasive coronary imaging significantly impact results and further progress in this field:
• There is a difference in methodology how to assess plaque burden between histology of Glagov and IVUS-imaging of Nissen;
• Neither intravascular intravascular ultrasound (IVUS) or optical coherence tomography nor noninvasive computed tomography angiography allow us to comprehensively distinguish all the artery layers;
• External elastic membrane and adventitia could be quantitatively assessed by IVUS only;
• Some new medications as well as progress in the development of the bioresorbable scaffolds and nanomedicine promise to revolutionize the cardiovascular biomedicine with the main goal to achieve Glagovian atheroregression below 40% threshold of the PB:
• The bioresorbable scaffold Absorb BVS (Abbott Vascular, CA, USA) is the first coronary device which has shown phenomena such as late lumen enlargement and wall thinning with at least 12% reduction of plaque burden;
• NANOM-FIM trial of plasmonic photothermal therapy with silica-gold nanoparticles showed truly unprecedented 60.3 mm3 plaque volume reduction.
• The modern-day statin trials were conducted in patients with relatively small lesions when PAV never exceeded 40% and without proper methodology, which means that the revealed phenomenon of atheroregression is essentially the pseudo reduction of the atheroma volume.
• The PAV or plaque burden is the only parameter to describe changes in the vessel geometry from the Glagov phenomenon point of view because it mathematically reflects patterns between both vessel and lumen size.
• IVUS with assessment of plaque burden remains the golden standard to evaluate atheroregressive patterns of medications or medical devices in clinical trials:
• Trials with the baseline PAV above 40% demonstrated higher potential of the plaque burden reduction;
• Further comprehensive analysis is required in order to validate the genuine threshold of the artery enlargement between a 20 and 55% PAV when positive correlation between lumen area and PB gets replaced by the negative correlation with the progressive narrowing of the lumen.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest.
- Nissen SE, Tuzcu EM, Brown BG et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 291, 1071–1080 (2004).
- Nissen SE, Tuzcu EM, Libby P et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled study. JAMA 292, 2217–2226 (2004).
- Tardif JC, Gregoire J, L’Allier PL et al. Effects of the acyl coenzyme A: cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation 110, 3372– 3377 (2004).
- Okazaki S, Yokoyama T, Miyauchi K et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH study. Circulation 110(9), 1061–1068 (2004).
- Nissen SE, Nicholls SJ, Sipahi I et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 295, 1556–1565 (2006).
- Nissen SE, Nicholls SJ, Wolski K et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299(13), 1561–1573 (2008).
- Hiro T, Kimura T, Morimoto T et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a milticenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS study). J. Am. Coll. Cardiol. 54(4), 293–302 (2009).
- Takayama T, Hiro T, Yamagishi M et al. Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Circ. J. 73(11), 2110–2117 (2009).
- Hirayama A, Saito S, Ueda Y et al. Plaque-stabilizing effect of atorvastatin is stronger for plaques evaluated as more unstable by angioscopy and intravenous ultrasound. Circ. J. 75(6), 1448–1454 (2011).
- Nicholls SJ, Ballantyne CM, Barter PJ et al. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 365, 2078–2087 (2011).
- Räber L, Taniwaki M, Zaugg S et al. Effect of high-intensity statin therapy on atherosclerosis in non-infarct-related coronary arteries (IBIS-4): a serial intravascular ultrasonography study. Eur. Heart J.36(8), 490–500 (2015).
- Nissen SE, Tsunoda T, Tuzcu EM et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 290, 2292–2300 (2003).
- Serruys PW, Garcia-Garcia HM, Buszman P et al. Effects of the direct lipoprotein-associated phospholipase A2 inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 118, 1172–1182 (2008).
- Nakajima N, Miyauchi K, Yokoyama T et al. Effect of combination of ezetimibe and a statin on coronary plaque regression in patients with acute coronary syndrome ZEUS trial (eZEtimibe Ultrasound Study). IJC Metab. Endocr. 3, 8–13 (2014).
- Martinet W, De Loof H, De Meyer GRY. mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis 233, 601–607 (2014).
- Onuma Y, Muramatsu T, Kharlamov A, Serruys PW. Freeing the vessel from metallic cage: what can we achieve with bioresorbable vascular scaffolds? Cardiovasc. Interv. Ther. 27, 141–54 (2012).
- Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2 year outcomes and results from multiple imaging methods. Lancet 373, 897–910 (2009).
- Kharlamov A, Gabinsky J. Plasmonic nanophotothermal and stem cell therapy of atherosclerotic plaque as the novel tool for angioplasty and artery remodeling. Rejuvenation Res. 15, 222–230 (2012).
- Kharlamov AN, Tyurnina AE, Veselova VS, Kovtun OP, Shur VY, Gabinsky JL. Silica-gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial. Nanoscale 7, 8003–8015 (2015).
- Kharlamov AN. Plasmonic photothermal therapy for atheroregression below Glagov threshold. Future Cardiol. 9(3), 405–425 (2013).
- Yeager D, Chen YS, Litovsky S, Emelianov S. Intravascular photoacoustics for image-guidance and temperature monitoring during plasmonic photothermal therapy of atherosclerotic plaques: a feasibility study. Theranostics 4(1), 36–46 (2013).
- Glagov S, Weisenberg E, Zarins CK et al. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 316, 1371–1375 (1987).
- Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler. Thromb. Vasc. Biol. 27, 1722–1728 (2007).
- Joner M, Virmani R. Glagov’s phenomenon: has our understanding of vascular remodeling changed? Coron. Artery Dis. 25(2), 91–93 (2014).
- Kruk M, Wardziak L, Mintz GS et al. Accuracy of coronary computed tomography angiography vs intravascular ultrasound for evaluation of vessel area. J. Cardiovasc. Comput. Tomogr. 8, 141–148 (2014).
- Kharlamov AN, Duckers HJ, van Beusekom HMM et al. Do we have a future with transcatheter adventitial delivery of stem cells? Int. J. Cardiol. 165, 217–221 (2013).
- Ward MR, Pasterkamp G, Yeung AC. Arterial remodeling: mechanisms and clinical implications. Circulation 102, 1186–1191 (2000).
- Serruys PW, Onuma Y, Muramatsu T et al. Dynamics of vessel wall changes following the implantation of the absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study at 6, 12, 24 and 36 months. EuroIntervention 9(11), 1271–1284 (2014).
- Gogas B. Bioresorbable scaffolds for percutaneous coronary interventions. Glob. Cardiol. Sci. Pract.2014(4), 409–427 (2014).
- Narula J, Nakano M, Virmani R et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J. Am. Coll. Cardiol. 61(10), 1041–1051 (2013).
- Virmani R, Kolodgie FD, Burke AP et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275 (2000).
- Gao WQ, Feng QZ, Li YF et al. Systematic study of the effects of lowering low-density lipoprotein-cholesterol on regression of coronary atherosclerotic plaques using intravascular ultrasound. BMC Cardiovasc. Disord. 14, 60 (2014).
- Tian J, Gu X, Sun Y et al. Effect of statin therapy on the progression of coronary atherosclerosis. BMC Cardiovasc. Disord. 12, 70 (2012).
- Zeb I, Li D, Nasir K et al. Effect of statin treatment on coronary plaque progression - a serial coronary CT angiography study. Atherosclerosis 231, 198–204 (2013).
- Koskinas KC, Windecker S, Räber L. Regression of coronary atherosclerosis: current evidence and future perspectives. Trends Cardiovasc. Med. doi: 10.1016/j.tcm.2015.05.004(2015) (Epub ahead of print).
- DuBroff R, de Lorgeril M. Cholesterol confusion and statin controversy. World J. Cardiol. 7(7), 404–409 (2015).
- Banach M, Serban C, Sahebkar A et al. Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med.13(1), 229 (2015).