Perspective - Imaging in Medicine (2013) Volume 5, Issue 2
Will CT colonography improve colorectal cancer screening compliance?
Mark N Damiano1 & Brooks D Cash*11Gastroenterology Service, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Building 1, Room 5151, Bethesda, MD 20889, USA
- Corresponding Author:
- Brooks D Cash
Gastroenterology Service
Walter Reed National Military Medical Center 8901
Wisconsin Avenue, Building 1, Room 5151
Bethesda, MD 20889, USA
Tel: +1 301 295 4585
Fax: +1 301 295 4599
E-mail: brooks.d.cash.health@health.mil
Abstract
Keywords
colonoscopy ▪ colorectal cancer ▪ CT ▪ CT colonography ▪ HEDIS® ▪ screening
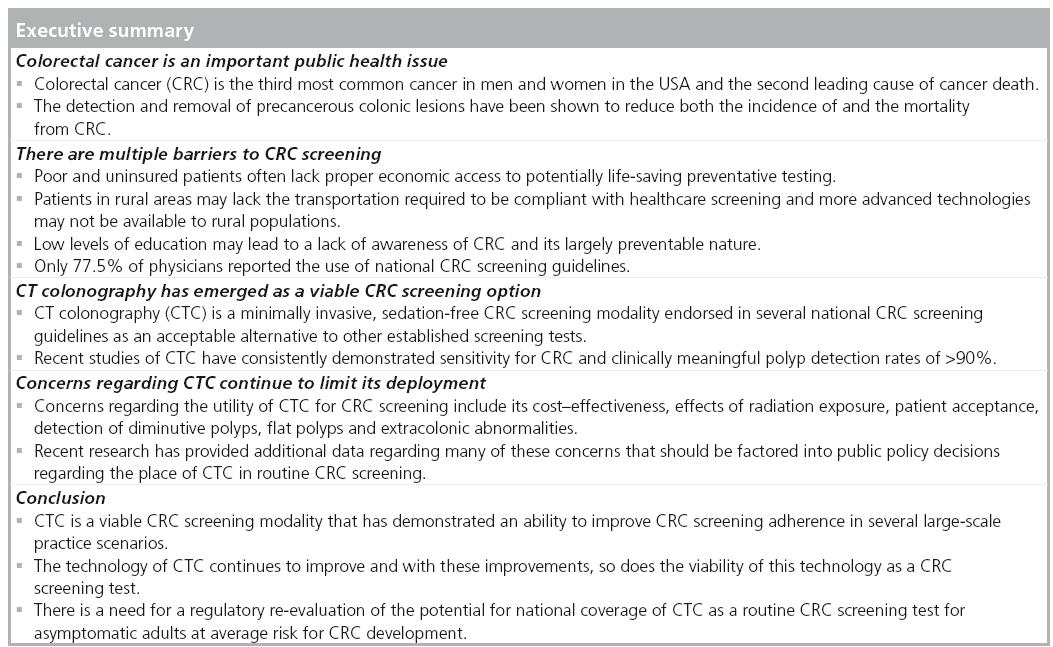
Colorectal cancer (CRC) is the third most common cancer in men and women in the USA and is the second leading cause of cancer death [201]. In 2012, it was estimated that 143,460 new cases of CRC will be diagnosed and that 51,690 people would die from complications related to this disease [202]. It has long been accepted that CRC generally begins as a benign precursor lesion, the adenomatous polyp, which develops slowly into a malignancy [1]. The time required for an adenomatous polyp to transform into a malignant lesion has been estimated between 8 and 15 years [2]. This prolonged latency period provides a unique opportunity for actual cancer prevention through the timely detection and removal of the obligate precursor adenomatous polyp. Unlike breast, lung and prostate cancers, whose screening methods focus on detecting already existing cancers at an early stage, CRC screening focuses primarily on detecting precancerous lesions. As would be expected, the detection and removal of precancerous colonic lesions has been shown to reduce both the incidence of and the mortality from CRC [3,4]. The 2009 Annual Report to the Nation on the Status of Cancer reported that increased screening represented the single largest contribution to the marked reductions in the incidence and mortality of CRC over the last two decades [5]. However, despite the widespread publication of evidence-based, expert consensus guidelines for the screening and surveillance of CRC, the overall adherence to CRC screening recommendations remains suboptimal. While CRC screening rates have increased since the guidelines were first introduced in the mid- 1990s, the CDC have reported that in 2010, only 59% of men and women reported having undergone recent CRC screening. This lags significantly behind the use of mammography for breast cancer screening, as well as the use of Papanicolau testing for cervical cancer screening, wherein close to three-quarters (72%) of women report being up-to-date [6]. It is estimated that approximately 42 million Americans between the ages of 50–75 years have not been screened for CRC [203]. Despite screening rates among individuals in this age group increasing from 52.3 to 65.4% between 2002 and 2010, over one-third of this at-risk population remains unscreened [7]. As would be expected, among the uninsured/underserved, screening rates are significantly worse, with a compliance of barely greater than 20%.
In addition to substandard compliance with CRC screening as a whole throughout the USA, there also appears to be a geographical variation. A recent review of the Surveillance, Epidemiology and End Result (SEER) mortality database demonstrated regional disparity in the rates of decrease in CRC mortality. The decrease in death rates between 1990 and 1994, and 2003 and 2007 were greater than 33% in northeastern states, such as Massachusetts and New York, but were only 9% in southern states, such as Alabama, while there was no decrease detected in Mississippi [8]. The authors surmised that this apparent difference in the mortality rates reflects a difference in CRC screening rates among these regions due to southern states having an increased population of poor and uninsured patients, as well as a higher percentage of African–Americans in whom CRC has a higher incidence and presentation at a more advanced stage [201]. Any change that leads to a significant improvement in adherence to CRC screening recommendations has the potential to have a dramatic impact on the incidence and mortality of CRC.
Barriers to CRC screening
Many barriers to compliance with CRC screening recommendations exist, some of which relate to screening in general, while others are test specific. Poor and uninsured patients often lack proper economic access to potentially lifesaving preventative testing. Patients in rural areas may lack the transportation required to be compliant with healthcare screenings and more advanced technologies may not be available to rural populations. Low levels of education may lead to a lack of awareness of CRC and its largely preventable nature. A recent study demonstrated that patients with low literacy have been found to be less aware of advertisements promoting CRC screening, less likely to believe that CRC screening was helpful and less likely to be compliant with recommended screening [9]. Other patient-specific barriers may include a fatalistic sense of fear or denial, a perceived lack of social support, only seeking healthcare when sick, and maintenance of a low level of concern due to a lack of symptoms and a lack of CRC in the family [10].
The medical field also has the potential, at times, to serve as a barrier to CRC screening. In one study of primary care providers, only 77.5% of physicians reported the use of national CRC screening guidelines [11]. Furthermore, only 51.7% reported that their recommendations were consistent with the guidelines. Interestingly, the manner in which a provider recommends CRC screening also has the potential to impact adherence with recommendations. Many providers equate ‘colon cancer screening’ with ‘colonoscopy’ and thus only offer patients this one option. Others may be motivated by economic considerations. Research suggests that as few as 40% of patients referred for colonoscopy are even aware that alternative screening options exist [12]. Inadomi et al. performed a study of 997 patients in which they were offered a colonoscopy, a fecal occult blood test (FOBT), or given a choice between the two methods. They found that participants who received a recommendation to undergo colonoscopy adhered to screening at a significantly lower rate (38%) than participants who received a recommendation for screening with FOBT (67%), as well as those given a choice between FOBT and colonoscopy (69%) [13]. This was especially true among racial/ethnic minorities and suggests that patient preferences and backgrounds should be considered when offering CRC screening recommendations and that giving patients multiple options and the ability to choose their CRC screening modality might lead to increased compliance.
CRC screening
Currently recommended methods of CRC screening include the use of high-sensitivity FOBTs or fecal immunochemical testing annually, double-contrast barium enema every 5 years, flexible sigmoidoscopy every 5 years, CT colonography (CTC; also known as virtual colonoscopy) every 5 years and optical colonoscopy (OC) every 10 years. However, after the Consolidated Appropriations Act of 2001 made screening colonoscopy available to Medicare beneficiaries, whole-bowel inspection by OC emerged as the preferred CRC screening method in the USA and came to be considered the ‘gold standard’ for CRC screening and prevention in this country [4,14].
While colonoscopy with polypectomy has clearly led to a reduction in the incidence and mortality of CRC, it remains an imperfect gold standard. There are multiple test-specific barriers that exist with OC including its invasive nature, the unpleasant preparation inherent in cleansing the bowel, the need for sedation/anesthesia, which is associated with a small, but measurable, complication rate, the embarrassing nature of the procedure, the potential for pain, the amount of time required for the preparation and the examination, and the need to provide an escort for conveyance to and from the procedure. A recent study examining barriers to CRC screening in a racially diverse, low-income population found that patients who did not follow through with obtaining their recommended CRC screening tended to have more issues with scheduling colonoscopy and with finding transportation compared with those patients who completed their screening [15]. Barriers regarding fear of pain and risk of injury tended to affect noncompleters more so than completers; however, this result was not statistically significant.
There are also several procedural risks inherent to OC, including bleeding, infection, perforation and sedation-associated cardiopulmonary complications. It has been estimated that approximately 1% of patients who undergo colonoscopy will require an emergency room visit or hospitalization as a result of procedural complications [16]. The rate of perforation with colonoscopy reported in large studies is 0.3% or less and is generally <0.1% [17], but is associated with a 5–7% risk of death [18,19]. Over 85% of serious colonoscopy complications are reported in patients undergoing colonoscopy with polypectomy [20,21]. An analysis of Canadian administrative data, including over 97,000 colonoscopies, found that polypectomy was associated with a sevenfold increase in the risk of bleeding or perforation [22]. Extrapolating these numbers using 2008 Medicare utilization data, one can estimate that approximately 300 Medicare patients can be expected to die annually from complications of colonic perforation alone. While the absolute risk may be small to any one individual, the risks and invasiveness of OC likely contribute to the suboptimal compliance with CRC screening recommendations and may partially explain why adherence lags behind screening for other cancers.
CT colonography
CTC is a minimally invasive, sedation-free CRC screening modality endorsed in several national CRC-screening guidelines as an acceptable alternative to other established screening tests. A low-radiation dose helical CT scan without use of intravenous contrast is performed and, with the aid of sophisticated postprocessing software tools, a 3D model of the colon is created, which allows the reader to ‘fly-through’ the cleansed, distended colon in both antegrade and retrograde directions. The entire 3D model can also be correlated with the conventional 2D CT scan images. Alternatively, a primary 2D approach can be used for the initial interpretation, with 3D images used for problem solving. While CTC is associated with lower risks of various complications compared with colonoscopy, bowel perforations have been reported with CTC, with an incidence of 0.06–0.08% [23]. Patients undergoing CTC who are referred to colonoscopy are subject to the same complications of that procedure discussed previously and appear to be at increased risk compared with a screening colonoscopy cohort due to the fact that they are more likely to undergo polypectomy of large polyps [24]. However, since the minority of patients undergoing screening CTC are referred to colonoscopy, the overall perforation rate with this modality is lower than that of colonoscopy.
CTC is indicated for both diagnostic and screening purposes. Screening indications for CTC include patients who are 50 years or older and are deemed to have an average risk of CRC [4]. There are multiple diagnostic indications for CTC, the most common of which is for patients who require completion of their CRC screening following an incomplete OC. In this situation, CTC is an excellent complementary test as the proximal colon, especially the cecum, is well distended during CTC and, thus, is very well visualized [25,26]. This indication has been supported since 2004 in 47 states within the USA [27]. Other diagnostic indications for CTC, reimbursed variably across the USA, include patients who are at high risk for OC-related complications (e.g., patients on anticoagulation medication or those with anesthesia risks) and patients who require evaluation of submucosal lesions detected during OC.
Likely owing to the current reimbursement issues, only a few centers have large CTC screening programs. In 2004, a congressional grant established the Colon Health Initiative at the National Naval Medical Center (now known as the Walter Reed National Military Medical Center) in Bethesda (MD, USA). The Colon Health Initiative is comprised of a dedicated team of radiologists, gastroenterologists, nurses, technologists and research personnel whose goal is to provide a multidisciplinary colon healthcare program with integrated clinical research for Department of Defense (DoD) medical beneficiaries in the National Capitol Region. It was at this facility that President Barack Obama underwent CTC screening in 2010 [204]. On the strength of the initial DoD results, medical directors of the major third-party payers in the Madison area (WI, USA) agreed to cover CTC for average risk screening performed at the University of Wisconsin Hospital and Clinics (WI, USA). Both institutions combined have performed over 10,000 CTC examinations since 2004.
The bowel-cleansing technique used for CTC is similar to that used for OC. Patients consume a standard low-volume bowel-cleansing preparation on the day prior to the procedure, consisting of either magnesium citrate and bisacodyl or a 2 l polyethylene glycol solution alone. In addition, single doses of 2% barium sulfate and diatrizoate sodium (Gastroview™, Covidien, MO, USA) alone or in combination are used for stool and colonic fluid tagging, respectively. Spasmolytics are not routinely used for CTC in the USA. Immediately prior to imaging, a small, flexible catheter is inserted into the rectum and the colon is insufflated via an automated carbon dioxide delivery system that is both volume and pressure regulated. Various center-specific insufflation protocols exist regarding the insufflation pressures and rates until full colonic distension is achieved, with some centers using lower and slower insufflation pressures and rates, whereas others initiate and maintain insufflation at a consistent, slightly higher pressure. Patients have reported no discomfort or only slight discomfort more often with the gentler insufflation approach (84%) compared with the more aggressive approach (47%) [28]. Once a scout film demonstrates adequate colonic insufflation, patients undergo multidetector CT imaging in both the supine and prone positions using a low-radiation dose protocol. Additional decubitus views may be necessary in patients with persistent luminal collapse. The imaging data are then transferred to dedicated CTC workstations for display and interpretation by experienced radiologists.
CTC performed without bowel purgation cleansing is a promising extension of the CTC technique in which small amounts of tagging agents are ingested 1–2 days prior to the examination in order to allow digital subtraction of labeled stool and fluid without a large volume catharsis [29–32]. A recent study from The Netherlands demonstrated excellent and comparable accuracy of a limited preparation CTC to a subsequent lavage-prepared colonoscopy in patients with positive occult blood tests [33]. This technique cannot currently be recommended as no large clinical studies in a screening population have validated its performance; however, it is reasonable to expect that continued technical and procedural refinements in the future may lead to ‘prepless’ CTC.
CTC efficacy
The early clinical trials of CTC in the late 1990s and early 2000s, which sought to evaluate the efficacy of CTC to detect large polyps and cancers, as well as to compare its performance with that of OC, yielded mixed results. In a study performed at Boston University (MA, USA), Fenlon et al. found that when using OC as the reference standard, CTC had a 100% sensitivity to detect cancers, 91% sensitivity to detect polyps 10 mm and larger, and 82% sensitivity to detect small polyps (6–9 mm) [34]. A larger trial performed at the University of California, San Francisco Veterans Administration (CA, USA) similarly showed that the sensitivity for detecting cancer was 100%, with 90% sensitivity to detect polyps ≥10 mm and 80% sensitivity to detect polyps 5–9 mm in size [35].
While these early trials yielded positive results, two larger trials published from 2003 to 2005 were less favorable. Johnson et al. performed a single-center trial using 2D image display techniques for lesion detection and noted a wide variation in the results of three different readers [36]. Sensitivities to detect 5–9 mm polyps and those ≥10 mm were found to be 41–69% and 35–72%, respectively. Rockey et al. performed a multicenter trial evaluating the diagnostic performance of CTC, air contrast barium enema and OC in which CTC per patient sensitivities to detect 6–9 mm polyps and ≥10 mm polyps were 51 and 59%, respectively [37]. There are several possible explanations for these less promising results. The early trials of CTC involved higher risk cohorts with a variety of procedural indications; evaluated the detection of all polyps as opposed to focusing solely on adenomas; largely relied on 2D image display techniques; and used CT scanners with spatial resolution that was inferior to that in more recent trials.
Around the same time that these studies were published, new technological advances were being exploited, which led to a landmark successful trial in the largest screening cohort of asymptomatic patients up to that point. Pickhardt et al. performed a multicenter trial evaluating 1233 asymptomatic patients for CRC screening with CTC [38]. Several novel techniques were introduced in this trial, including stool tagging with electronic subtraction, as well as 3D fly-through as the primary image display and interpretation technique. Furthermore, it used an enhanced reference standard of segmental unblinding of CTC results during OC in all patients. This technique involves the colonoscopist evaluating each colonic segment initially unaware of the CTC results, followed by a second look if the disclosed CTC results demonstrated a significant lesion. This trial reported per patient sensitivities to detect adenomas ≥6 and ≥10 mm of 88.7 and 93.8%, respectively. A subsequent analysis of the segmental unblinding data evaluated the miss rates of the initial OC, which were found to be 10% for adenomas larger than 10 mm [39]. As a result of this study, a new benchmark for improved diagnostic performance for the detection of polyps 6 mm and larger by CTC in screening cohorts was established.
In 2008, the ACRIN 6664 trial became the largest screening trial of CTC with 2531 asymptomatic patients at average risk for CRC involving a total of 15 centers in both academic and private practice settings [40]. In addition to 2D and 3D image display techniques and stool tagging, this trial also imposed standards for the qualifications of radiologists interpreting the CTC results. They found a per patient sensitivity for detecting polyps ≥10 mm of 90% with a 78% per patient sensitivity for detecting polyps ≥6 mm. While these results were more modest than the study by Pickhardt et al., the diversity of the centers in both academic and private practice settings is more likely to be representative of results expected in general practice.
Two additional studies further validated the efficacy of CTC to detect colorectal neoplasia. The IMPACT trial, which was performed in higher risk cohorts, showed that per patient sensitivity to detect polyps 6–9 mm and ≥10 mm was 85 and 91%, respectively [41]. In Germany, the Munich Colorectal Cancer Prevention Trial trial showed per patient sensitivities to detect polyps ≥6 and ≥10 mm in size to be 91 and 92%, respectively [42]. These results were lower than those achieved with OC at 98 and 100%, but much better than other approved CRC screening modalities including flexible sigmoidoscopy (67 and 68%), FOBT (18 and 24%) and fecal immunochemical testing (40 and 33%).
Most recently, results derived from large-volume CTC programs demonstrate that CTC has a similar accuracy to OC for the detection of CRC and clinically important colonic neoplasia [43]. In 2011, a comprehensive meta-analysis of CTC and OC for the detection of CRC described the results obtained in 11,151 patients from 49 studies conducted between 1994 and 2009 [44]. The sensitivity of CTC for detecting CRC was 96.1%. When both cathartic and stool tagging agents were used as part of the bowel preparation, CTC did not miss any cancers. In a subset of 25 studies involving 9223 patients, the sensitivity for OC to detect CRC was 94.7%. As more and more evidence mounts, it is clear that, similar to OC, CTC has a high sensitivity for the detection of colorectal cancer and polyps ≥6 mm in diameter.
Concerns regarding CTC
Despite convincing evidence that CTC has the potential to be extremely efficacious for the detection of CRC and clinically significant colonic neoplasia, there have been several concerns raised about the use of CTC that have limited its widespread adoption in the USA.
■ Flat lesions
Flat colorectal lesions represent a challenging problem for both OC and CTC. The reported prevalence of these lesions and the sensitivity for CTC to detect them has varied in the literature. Some of this variability is likely due to recent technological improvements in CTC software and CT spatial resolution leading to an increased ability to detect these lesions; however, differences in definitions of the morphology and terminology of flat lesions have also likely had an impact.
When defining a ‘sessile’ polyp as one in which the height of the lesion was less than half of the length, Fidler et al. reported a sensitivity for CTC to detect sessile lesions of less than 50% [45]. In a subsequent report, Pickhardt et al. performed an analysis of sessile lesions from the DoD experience and found a sensitivity of 83% [46]. Differing terminology was used by Soetikno et al. by which ‘polypoid’ lesions were defined as sessile or pedunculated in morphology, while ‘nonpolypoid’ lesions were defined as superficially elevated, flat or depressed [47]. In a series of CRC screenings of veterans using OC, the overall prevalence of nonpolypoid neoplasia was reported to be 9.4% and the prevalence in the screening subpopulation was reported as 5.8%. The concern that this report created regarding the possibility of failed detection of nonpolypoid lesions with OC was quickly extended to CTC as well. However, all CTC trials reported to date that have used OC as a gold standard have not described a significant trend of false negatives for flat or nonpolypoid lesions with CTC. Moreover, this morphological lesion is well recognized in the CTC community and is a part of the standardized training for CTC interpretation.
The Paris classification of flat lesions defines these lesions as being less than 3 mm in height [48]. A subcategory of the flat lesion is the carpet (or laterally spreading) lesion, which spans a distance of at least 3 cm. Using this terminology, Pickhardt et al. published a series evaluating over 5000 consecutive asymptomatic patients who underwent screening for CTC [49]. All lesions >6 mm in size were labeled as sessile or pedunculated (these were combined and called polypoid lesions) versus flat. Lesions >3 cm in length that were flat were labeled as carpet lesions. Among 954 polyps measuring ≥6 mm detected by CTC, 125 lesions (13.1%) in 106 adults were found to be flat lesions with a mean size of 12.7 mm. This included ten lesions >3 cm in length that were labeled as carpet lesions. For all flat lesions between 6 and 30 mm in size, the maximal height averaged 2.2 mm and was ≤3 mm in 86% of these lesions. Among the nine flat lesions seen only with OC (false negatives on CTC), only two were neoplastic (tubular adenomas) and none were histologically advanced. As this study shows, CTC is quite adept at the detection of clinically relevant flat lesions and future improvements in CT acquisition parameters, computer aided diagnosis software and 3D image display techniques should be expected to continue to improve the detection of this morphological type of colorectal lesion.
■ Diminutive colorectal polyps
The benign nature of diminutive (≤5 mm) colorectal polyps is generally accepted. In a study published by Butterly et al., the prevalence of advanced histology in diminutive polyps was reported to be 1.7%, while the prevalence of carcinoma in these lesions was 0% [50]. Despite this reassuring evidence, there has been concern voiced that CTC has a diminished sensitivity for the detection of diminutive lesions.
The anxiety surrounding the clinical importance of diminutive polyps left undetected or unreported after CTC is understandable. Colonoscopists are taught to remove all polyps that are identified, even though more than 90% of the polyps detected during colonoscopy are small (6–9 mm) or diminutive (≤5 mm) and only about half of these polyps are neoplastic. Removal of these lesions during colonoscopy increases both the risk of adverse events due to polypectomy as well as the cost associated with colonoscopy screening, with additional charges for performing polypectomy, endoscopic resection equipment and pathology processing and interpretation. Recent endoscopic research has called into question the necessity of removing all polyps seen during colonoscopy based on advances in endoscopic imaging and the potential for arriving at a real-time histologic diagnosis that would allow polyps to be left resected and discarded in vivo [51]. Nevertheless, Shah et al. demonstrated that the anxiety associated with not knowing polyp histology is considerable for clinicians and patients [52]. Although the literature suggests a very small risk of advanced histology or cancer in diminutive colonic polyps, there is a nontrivial risk of approximately 6–8% in small polyps [53]. In the DISCARD trial, 14 advanced adenomas ≤10 mm were identified in nine patients (6.9%) [51]. According to the ‘remove and discard’ policy used in this trial, 77% of these patients would have been given inappropriately extended surveillance interval recommendations had the polyps not been sent for confirmatory histologic examination. Moreover, the natural history of these lesions is not known with any degree of certainty. The practice of leaving polyps in vivo, although attractive in terms of reducing costs and complications, could prove to be unacceptable to the consumer based on our limited knowledge of their natural history.
CTC may actually permit advances in our understanding of the natural history of colonic polyps. In a study examining the natural history of small colorectal polyps, Pickhardt et al. reported that volumetric growth rates were highly predictive of advanced histology [Pickhardt PJ et al., Pers. Comm.]. Using a baseline threshold of ± 20% change in annual polyp volume, they reported that 22% of small polyps progressed in size while 50% remained stable and 28% actually regressed during CTC surveillance. All 24 advanced adenomas showed positive volume growth at follow-up. The sensitivity and specificity of CTC to detect advanced adenomas in this study was 92 and 94%, respectively. Given the 5-year interval currently recommended after a negative CTC and the long latent period of polyp growth, a diminutive polyp showing positive growth behavior, as is seen in advanced adenomas, could theoretically be identified and referred for polypectomy while still in a benign stage of development. Furthermore, the practice of tracking diminutive polyps for growth via CTC surveillance as opposed to immediate referral for polypectomy ought to lead to the appropriate removal of advanced adenomas, one of the primary targets of CRC screening, while avoiding the risks and costs associated with the removal of clinically insignificant neoplastic colorectal lesions.
■ Radiation exposure during CTC screening
Some modeling studies have estimated that up to 1% of the malignancies in the USA could be attributed to medical radiation exposure [54], and cumulative radiation exposure due to medical imaging has been a topic of major concern over the past several decades as medical imaging frequency has increased dramatically [55]. Recently, the American College of Radiology created a Blue Ribbon Panel on Radiation Dose in Medicine and published recommendations and quality initiatives for the safe use of ionizing radiation, including CT, in clinical practice [56]. Several recent reports have addressed the issue of low-dose radiation exposure with CTC. Brenner and Georgsson concluded that the benefit-to-risk ratio with CTC was high and that radiation-induced cancer risks were very low [57]. They also concluded that the potential lifetime cancer risk for one CTC exam at age 50 years was 0.14% (0.07% at age 70 years), while noting that this risk could be reduced by factors of five or ten with optimized low-dose radiation protocols.
Unlike routine abdominal CT, which identifies solid-organ abnormalities using differences in x-ray attenuation between soft tissue structures, CTC identifies colonic polyps and cancers by exploiting the attenuation difference between the these soft tissue lesions and intracolonic gas. The resulting attenuation gradient is much greater, permitting CTC exams to be performed at much lower radiation doses. Scanning at lower dose (i.e., lower mAs settings, higher pitch) increases image noise and complicates visualization of extracolonic structures, but does not compromise the detection of colorectal polyps and cancers 5 mm or greater in size [58–62]. A study from 2003 investigated the use of low-dose CTC and its efficacy for detecting colorectal neoplasia [60]. A cohort of 158 patients at a predominantly increased risk of CRC underwent CTC using a low dose of 10 mAs and a slice thickness of 2.5 mm. This protocol resulted in total effective doses of 1.8 mSv in men and 2.4 mSv in women. By comparison, the effective radiation dose for a barium enema and a standard CT scan of the abdomen is approximately 7 and 10 mSv, respectively. Using this low-dose radiation protocol, the sensitivity for detecting 22 CRCs was 100%, the sensitivity for 13 polyps greater than 1 cm in size was 100%, and the sensitivity for detecting polyps from 6 to 9 mm was 83%.
Additional decreases in radiation dose have since been achieved due to advances in automatic tube current exposure and dose modulation techniques, which differentially change the delivered dose over the anatomy scanned in real time (e.g., more of the radiation dose is given to penetrate the bony pelvis and less is given over the soft tissues of the abdomen) [63]. Utilizing these new dose-reduction techniques, the effective radiation dose from CTC contributes minimally to the yearly background radiation. These low-dose radiation techniques have become the standard of care for screening with CTC in both research and clinical practice. Even more recent estimates of dose delivery associated with CTC place the effective dose between 1–2 mSv [41,64]. Nevertheless, some countries in Europe have prohibited the use of radiation modalities for screening, so it is clear that radiation exposure and the unknown risks associated with radiation remain a concern [23].
■ CTC interpretation quality
Several early CTC studies mentioned previously that showed suboptimal efficacy for detecting CRC and advanced neoplasia were criticized due to the lack of rigorous training and experience of the radiologists interpreting the studies [65]. This has led to the concern that widespread availability of CTC could potentially lead to an unacceptable number of missed lesions due to reader inexperience and a lack of appropriately trained radiologists. This need not be the case. Larger medical centers can identify excellent radiologists who can focus on reading large volumes of CTC images and deliver high-quality interpretations. The increased availability of teleradiology can permit smaller centers or practices to send CTC images to major academic centers or dedicated high-quality CTC interpreters for remote readings. In one such report, Friedman and colleagues successfully implemented a CTC program for underserved communities in rural Arizona [66]. Three hundred and twenty one subjects underwent CTC at two rural hospitals with transmission of images to CTC interpreters at the University of Arizona (AZ, USA), including 280 individuals referred for screening examinations (87%). A total of 92% of subjects (295 out of 321) had acceptable amounts of residual stool, 91% (293 out of 321) had acceptable levels of fluid and 92% (294 out of 321) had acceptable distention. A total of 14% (44 out of 321) of CTC patients had polyps ≥6 mm in size, with a positive predictive value of 41% for those who subsequently underwent colonoscopy with polypectomy. In addition, while the time commitment to develop CTC interpretive expertise is substantial and the maintenance of proficiency requires ongoing education and training, adequately trained gastroenterologists have been able to interpret colonic CTC images with proficiency similar to that of radiologists [67].
Modifications in software analysis/ rendering of images, including so-called ‘virtual dissection’ and computer-aided detection may facilitate the implementation of CTC [68–71]. Computer-aided detection in particular appears to be a major potential advance. In this technique, data sets are generated from transverse CT sections and volumetric features characterizing polyps are computed. Polyps can then be detected by means of sophisticated thresholding algorithms followed by mathematical rule-based testing on the basis of feature values [72–75]. While this technique appears to hold great promise and will likely be readily integrated into reading schemes [76,77], many issues with regard to implementing computer- aided detection into clinical practice remain.
■ Multiple bowel preparations
There are many patients who report that the worst part of undergoing an OC is consuming the prescribed bowel preparation. Thus, many patients have the valid concern that undergoing a CTC might necessitate having to go through the bowel preparation process twice if a clinically significant lesion is identified and same-day OC is not available. Patients should be appropriately counseled about this possibility and, for some, OC may be a better option to avoid this possibility. However, the overall referral rate for polypectomy after a CTC is relatively low. The University of Wisconsin reports a referral rate for therapeutic optical colonoscopy in older patients (ages 65–79 years) of approximately 15% [78]. This center’s referral rate for average risk patients has been estimated at approximately 8%. This equates to an average referral rate of 10–12%, which is similar to that of our high volume CTC center at the Walter Reed National Military Medical Center.
■ Extracolonic findings
One of the potential benefits of using cross-sectional body imaging during CTC is the potential to diagnose unsuspected, clinically relevant extracolonic disease. This aspect of CTC does have the potential to be a risk if it were to expose patients to extensive subsequent noninvasive or invasive testing. Such testing would also result in additional healthcare costs. However, judicious management of extracolonic findings can balance the benefits of early detection of important diseases with the costs and risk associated with additional evaluation of extracolonic findings.
In an effort to provide a uniform approach to reporting extracolonic findings at CTC, a classification system called CT Colonography Reporting and Data System (C-RADS), which is analogous to the Breast Imaging Reporting and Data System (BI-RADS®) used for mammography, has been implemented [79]. Extracolonic findings of at least moderate potential clinical importance are assigned to either C-RADS category E4 (potentially important finding) or C-RADS category E3 (likely unimportant finding, incompletely characterized). All other incidental findings are assigned to C-RADS category E2 (clinically unimportant finding), while the lack of any notable findings would be classified as C-RADS category E1.
The rate of detecting extracolonic findings believed to be of at least moderate potential importance has been found to fall in a rather narrow range of 7.4–11.4% according to several published series [80–83]. At New York University (NY, USA), a recent retrospective evaluation of extracolonic findings in both senior and nonsenior patients reported the rate of potentially important extracolonic findings to be 8% [84]. In perhaps the largest study involving extracolonic findings, Pickhardt et al. evaluated 2195 consecutive asymptomatic adults and found that 189 (8.6%) patients had previously unknown extracolonic findings of at least moderate potential clinical importance [85]. Additional work-up was recommended or suggested by the radiologist in 157 (7.2%) patients and was ultimately undertaken in 133 (6.1%) of them. A total of 55 (2.5%) patients were diagnosed with an unsuspected condition of at least moderate importance. In this study, the estimated costs of additional diagnostic and therapeutic work up related to newly discovered extracolonic findings were US$98.56 per patient screened.
If CTC is to be employed for widespread CRC population screening, the potential impact from extracolonic findings becomes relevant from the standpoints of clinical effectiveness and cost–effectiveness. The vast majority of these extracolonic findings will ultimately prove to be of little or no clinical relevance (e.g., benign renal cysts or hepatic hemangiomas). The risk of an asymptomatic adult harboring a clinically important disease is low, but it is not zero. Pickhardt et al. reported encountering an unsuspected extracolonic malignancy at CTC in approximately one out of every 250 patients screened [86]. By comparison, their rate of encountering an unsuspected colorectal cancer during OC screening has been reported to be one out of every 400–500 patients screened. The judicious handling of extracolonic findings at CTC, as opposed to practicing ‘defensive medicine’, will be essential to maintain this benefit while minimizing the impact of unintended extracolonic findings in terms of patient risk and additional healthcare costs.
■ Integration of CTC into a CRC screening program
Incorporating CTC into an already efficient CRC screening program can be potentially challenging and requires coordination of several key processes, especially if same-day OC is to be offered. Duncan et al. recently reviewed the database of all CTCs performed from 2004 to 2010 at the National Naval Medical Center and found that 11% of patients required therapeutic OC due to positive findings on CTC [87]. Of the 1137 patients referred for OC after CTC, only 130 patients underwent a same-day procedure. The course of events that was required for sameday OC after a positive CTC included registration for CTC, performance of CTC, immediate CTC interpretation, endoscopy unit registration, OC preparation and completion, and postprocedural observation and discharge. As might be expected, the average wait time from arrival for CTC to discharge after OC was long at 348 min (range 178–684 min). Minimizing the total wait time for a patient undergoing same-day OC will be paramount for maintaining an efficient CRC screening program involving CTC.
In order to maintain and ensure high-quality CRC screening, several quality improvement measures need to be in place in order to provide meaningful feedback to physicians as they strive to improve either their endoscopic or radiologic expertise. Adenoma detection rates should be followed by both endoscopists and radiologists alike, and, therefore, close coordination with pathology is required for effective CTC and OC integration. In addition, the rate of E2 and E3 findings should be monitored and compared with national database statistics in order to reduce additional risk and healthcare costs, as previously mentioned.
There are limited data on the impact that widespread implementation of CTC might have upon OC volume at endoscopy centers. In a study published in 2008, Schwartz et al. noted that the total number of OC performed from 2004 to 2007 did not decrease at the University of Wisconsin after the initiation of a CTC screening program [88]. A mean of ten patients per month were referred for OC after a positive CTC. In fact, it was concluded that even after CTC was integrated into their CRC screening program, OC remained the predominant screening modality [89]. Perhaps most importantly, Ladabaum and Song noted that if overall adherence to CRC screening guidelines is attained by a population, then overall OC volume increases, even with the widespread use of CTC for screening [90].
■ Cost–effectiveness
A study by Kim and colleagues demonstrated some of the potential advantages of CTC with respect to cost and complications [91]. In this study, the screening test results of two parallel groups, both >3000 patients undergoing either primary CTC or colonoscopy, were compared. Similar rates of advanced neoplasia were found; however, the frequency of polypectomy was fivefold greater in the primary colonoscopy group. In addition, complications occurred in 0.2% of the colonoscopy group and none of the patients in the CTC group. Both of these differences were statistically significant. Only 7.9% of patients undergoing primary CTC progressed to colonoscopy, although it should be noted that some of the patients in the CTC group were participating in a polyp observation trial, so this value is artificially low. While there was no formal cost comparison preformed, the findings of this analysis clearly demonstrate the potential of CTC to lower the costs associated with polypectomy and complications from colonoscopy. However, other unknowns that could affect future costs, such as the natural history of nonremoved neoplasia or possible radiation-induced future diseases, would also need to be factored into future cost–effectiveness analyses.
A recent study evaluated the reimbursement rate at which CTC would be cost effective compared with other currently approved CRC screening modalities among the average-risk Medicare population [92]. It was found that the number of life-years gained from CTC screening (143–178 per 1000 65‑year-olds) was comparable with that of flexible sigmoidoscopy every 5 years along with annual FOBT and was slightly less than that of OC every 10 years. If CTC were to be reimbursed at slightly less than the expected reimbursement for OC without polypectomy, then CTC would not be cost effective. Using this model, CTC would have to be offered at a cost substantially lower per scan than OC in order to be cost effective. However, CTC became the most effective screening option in the scenario where 10% of individuals, who would have otherwise eschewed CRC screening, elected to undergo CTC. Furthermore, this analysis also demonstrated that if the widespread availability of CTC was able to entice 25% of the otherwise unscreened population to be screened for CRC, then CTC would in fact be cost-effective overall, even at the current rate of reimbursement.
Patient experience with CTC
CTC is a well-tolerated procedure that patients often view favorably compared with colonoscopy. Moawad et al. surveyed 250 patients undergoing CTC and found that 36% would have forgone CRC screening had CTC not been an available CRC screening choice [93]. Also of importance, it was noted that among patients who had undergone both OC and CTC, 95% (54 out of 57) indicated that they actually preferred their experience with CTC. Pooler et al. performed the largest study to date evaluating patient experience and preference regarding CTC [28]. A total of 1417 patients from three diverse settings (a large military treatment facility, a large academic hospital center and a community-based private practice) were surveyed and the results highlighted several important findings for both experience with CTC and CRC screening in general. More than 72% of patients undergoing their first CRC screening had delayed their screening beyond the recommended age of 50 years (45 years for African–Americans) by an average of 7 years [94]. This rate of delayed screening was highest in the community setting and among African–Americans. A total of 93% of patients reported either a ‘good’ or ‘excellent’ overall experience with CTC. Regarding test preference, 93% also stated that they would choose CTC as their next CRC screening method and 95% reported that they would recommend CTC to their family and friends who needed to undergo CRC screening. Of those patients who had experienced both OC and CTC, 86% preferred their experience with CTC. This surprisingly high level of preference for CTC over OC suggests that some of the advantages of CTC are important to patients and may be utilized to help overcome some of the aforementioned test-specific barriers that exist with OC. Perhaps the most important finding from this study was that approximately 30% of the patients surveyed would have definitely or likely refused CRC screening with OC had CTC not been available to them (8.4% ‘no’ and 21.4% ‘not sure’).
However, other studies of patient experience with CTC contradict the above experiences. Howard et al. conducted a discrete choice study to assess preferences of patients with clinical indications suspicious of CRC who experienced both CTC and colonoscopy as part of a diagnostic accuracy study in south Australia and found that colonoscopy was preferred over CTC based on the increased risk of a second procedure after CTC, the increased risk of missing cancers or polyps and increased CTC cost [95]. Ghanouni and colleagues evaluated the public perception of CTC and colonoscopy by performing discussion groups with 180 adults. These investigators found that CTC was favored on the parameters of invasiveness, extra-colonic evaluation and interference with daily life, whereas sensitivity, avoiding false positives and the capacity to remove polyps immediately were perceived to be important advantages of colonoscopy. Ultimately, there was no strong preference for either test, with 46% preferring colonoscopy versus 42% for CTC [96]. It is actually fitting that the preference of CTC or colonoscopy compared with each other is unclear or equivocal, since CTC is not meant to replace colonoscopy but rather to augment that test to increase CRC screening adherence.
Impact of CTC on adherence to CRC screening guidelines
It has been said that the best CRC screening test is the one that a patient is willing to undergo [97]. Given the need for ongoing screening over a 25–30-year time period, patients’ experiences, satisfaction and preferences will undoubtedly play pivotal roles in future screening adherence. As we have seen, it is estimated that approximately 42 million Americans between the ages of 50 and 75 years have not been screened for CRC [203]. It could take up to 10 years to screen all of these patients using either flexible sigmoidoscopy or OC, given the available capacity in the US healthcare system [98]. Unless other screening options become available to the US population at large, we will fail to achieve our Healthy People 2020 target goal of 71% screening compliance for CRC [99].
A recent NIH consensus panel on CRC noted that CTC is an important addition to the list of effective CRC screening tests and that “utilizing the full range of screening options” is the most effective avenue through which significant improvements in screening levels and reductions in CRC-related morbidity and mortality can be achieved [100]. Based on evidence demonstrating that CTC offers sufficient sensitivity and specificity for use as a screening test for the general risk population, a number of professional societies and health insurers have supported the addition of CTC to the range of screening options, including the American Cancer Society, the Multi-Society Task Force on Colorectal Cancer, the American College of Radiology and the Blue Cross Blue Shield Association’s Technology Evaluation Center [101]. Several large commercial health insurers now cover CTC and 26 states and the District of Columbia mandate that third-party payers cover the costs of this screening test [102].
The potential for CTC to increase the USA’s adherence to CRC screening guidelines is substantial. At the National Naval Medical Center and Naval Medical Center San Diego, two large tertiary care medical facilities already equipped with efficient CRC screening programs that boast above average screening compliance rates, the inclusion of CTC into the Healthcare Effectiveness Data and Information Set (HEDIS®) metric dramatically increased CRC screening compliance above the 90th percentile by 14.4–15.7% [103]. If this degree of increased compliance can be achieved in an overall healthy population that had excellent access to healthcare with a focus on preventive medicine such as DoD medical beneficiaries, then it would not be a stretch to suggest that the widespread availability of CTC could increase adherence to CRC screening guidelines by 25–30% in the USA overall.
Conclusion
Despite advances over the last several decades, CRC remains a major health concern in the USA with over 50,000 deaths expected this year alone. Even though overwhelming evidence demonstrates that screening leads to dramatic decreases in incidence and mortality from CRC, adherence to current screening guidelines remains suboptimal. CTC is a minimally invasive, nonsedated test that has demonstrated equivalent efficacy as OC for detecting clinically significant colorectal neoplasia and CRC, as well as one that patients have found to be convenient and largely painfree. Published evidence suggests that the majority of patients who have undergone both CTC and OC prefer the CTC experience and would recommend this test to others in need of CRC screening. The results of well-performed clinical trials has demonstrated that CTC can identify CRC and large polyps as well as OC; however, questions remain regarding the ability of CTC to identify flat and/or serrated lesions and the clinical significance of diminutive polyps not identified on CTC. While the integration of CTC into CRC screening programs at dedicated centers has been shown to result in a substantial increase in CRC screening compliance without overwhelming endoscopic capabilities, additional comparative effectiveness data is needed to inform policy-makers’ decisions regarding the appropriateness and feasibility of integration of this screening modality into routine, reimbursed practice.
Future perspective
There is little credible debate that CTC cannot provide images of the colonic lumen that are on par with colonoscopy and with a lower risk of procedural complications. However, CTC continues to face resistance and generate strong feelings against its widespread use. We have gained considerable understanding regarding outcomes achievable with CTC since the Centers for Medicare and Medicaid Services (MD, USA) declined to include it for coverage as a CRC screening test. This decision, while predicated on unknowns related to radiation exposure, extracolonic findings and the impact of CTC on CRC screening uptake, mirrored those of the US Preventative Services Task Force (USPSTF; MD, USA) during the last review of CRC screening modalities. The USPSTF is scheduled to review CRC screening tests again in the next 1–2 years. We believe that the data that they found lacking on their last review has now been substantiated and published in the peer-reviewed literature to a satisfactory degree. On its last review, this organization gave FOBT, flexible sigmoidoscopy and colonoscopy a grade A recommendation. This means that the USPSTF recommends these tests because they feel that there is a high certainty that the net benefit is substantial, based on the published literature. If the recommendation scheme remains the same for the next cycle of CRC screening test evaluations, as it should be expected to, we should have every expectation that CTC will also gain an ‘A’ rating from the USPSTF. Once that happens, the Centers for Medicare and Medicaid Services should be asked to re-evaluate their stance on CTC coverage and another reasonable expectation is that the Centers for Medicare and Medicaid Services will include screening CTC for coverage. This may become especially important as healthcare reform increases the number of Americans with coverage and expectations for routine health-maintenance services that could very well outstrip available colonoscopic resources. CTC, as well as other modalities, may be well positioned to fill the void. Aggressive planning and forecasting on how best to integrate and deliver these services should be started now. In addition, radiology and gastroenterology training programs should ensure that they can provide adequate education and experience covering the performance of CTC. Outcomes databases and quality parameters should be established now in expectations of greater demand of CTC in the coming years and additional research related to benefits and costs associated with CTC, as well as other CRC screening tests, should continue to be collected and analyzed in order to assist policy-makers arrive at informed decisions to optimize population health.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the Department of the Navy, Department of Defense or the US Government.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
&bul; •of considerable interest
- Vogelstein B, Fearon ER, Hamilton SR et al. Genetic alterations during colorectal tumor development. N. Engl. J. Med. 319, 525–532 (1988).
- Saif MW, Chu E. Biology of colorectal cancer. Cancer J. 16, 196–201 (2010).
- Winawer S, Fletcher R, Rex D et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale – update based on new evidence. Gastroenterology 124, 544–560 (2003).
- Levin B, Lieberman DA, McFarland B et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J. Clin. 58, 130–160 (2008).
- Edwards BK, Ward E, Kohler BA et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 116, 544–573 (2010).
- CDC. Cancer screening – United States, 2010. MMWR Morb. Mortal. Wkly Rep. 61(3), 41–44 (2012).
- CDC. Vital signs: colorectal cancer screening, incidence, and mortality – United States, 2002–2010. MMWR Morb. Mortal. Wkly Rep. 5, 1–6 (2011).
- Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol. Biomarkers Prev. 20, 1296–1302 (2011).
- Arnold CL, Rademaker A, Bailey SC et al. Literacy barriers to colorectal cancer screening in community clinics. J. Health Commun. 17(Suppl. 3), 252–264 (2012).
- Garcia-Dominic O, Lengerich EJ, Wray LA et al. Barriers to CRC screening among latino adults in pennsylvania: ACCN results. Am. J. Health. Behav. 36(2), 153–167 (2012).
- Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol. Biomarkers Prev. 15, 389–394 (2006).
- Denberg TD, Melhado TV, Coombes JM et al. Predictors of nonadherence to screening colonoscopy. J. Gen. Intern. Med. 20(11), 989–995 (2005).
- Inadomi JM, Vijan S, Janz NK et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch. Intern. Med. 172(7), 575–582 (2012).
- Centers for Medicare and Medicaid Services, HHS. Revisions to payment policies and fiveyear review of and adjustments to the relative value units under the physician fee schedule for calendar year 2002: final rule with common period. Fed. Regist. 66, 55246–55503 (2001).
- Quick BW, Hester CM, Young KL, Greiner KA. Self-reported barriers to colorectal cancer screening in a racially diverse, low-income study population. J. Community Health doi:10.1007/s10900-012-9612-6 (2012) (Epub ahead of print).
- Leffler DA, Kheraj R, Garud S et al. The incidence and cost of unexpected hospital use after scheduled outpatient endoscopy. Arch. Intern. Med. 170, 1752–1757 (2010).
- Ko CW, Dominitz JA. Complications of colonoscopy: magnitude and management. Gastrointest. Endosc. Clin. N. Am. 20, 659–671 (2010).
- Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J. Natl Cancer Inst. 95, 230–236 (2003).
- Iqbal CW, Cullinane DC, Schiller HJ, Sawyer MD, Zietlow SP, Farley DR. Surgical management and outcomes of 165 colonoscopic perforations from a single institution. Arch. Surg. 143, 701–706 (2008).
- Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann. Int. Med. 149, 638–658 (2008).
- Crispin A, Birkner B, Munte A, Nusko G, Mansmann U. Process quality and incidence of acute complications in a series of more than 230,000 outpatient colonoscopies. Endoscopy 41(12), 1018–1025 (2009).
- Rabeneck L, Paszat LF, Hilsden RJ et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology 135, 1899–1906 (2008).
- Pox CP, Schmiegel W. Role of CT colonography in colorectal cancer screening: risks and benefits. Gut 59, 692–700 (2010).
- Heldwein W, Dollhopf M, Rösch T et al. The Munich Polypectomy Study (MUPS): prospective analysis of complications and risk factors in 4000 colonic snare polypectomies. Endoscopy 37(11), 1116–1122 (2005).
- Copel L, Sosna J, Kruskal JB, Raptopoulos V, Farrell RJ, Morrin MM. CT colonography in 546 patients with incomplete colonoscopy. Radiology 244, 471–478 (2007).
- Neri E, Giusti P, Battolla L et al. Colorectal cancer: role of CT colonography in preoperative evaluation after incomplete colonoscopy. Radiology 223, 615–619 (2002).
- Knechtges PM, McFarland BG, Keysor KJ, Duszak R Jr, Barish MA, Carlos RC. National and local trends in CT colonography reimbursement: past, present, and future. J. Am. Coll. Radiol. 4, 776–799 (2007).
- Pooler BD, Baumel MJ, Cash BD et al. Screening CT colonography: multicenter survey of patient experience, preference, and potential impact on adherence. AJR Am. J. Roentgenol. 198(6), 1361–1366 (2012).
- Callstrom MR, Johnson CD, Fletcher JG et al. CT colonography without cathartic preparation: feasibility study. Radiology 219, 693–698 (2001).
- Lefere P, Gryspeerdt SS, Marrannes J, Baekelandt M, Van Holsbeeck B. CT colonography after fecal tagging with a reduced cathartic cleansing and a reduced volume of barium. AJR Am. J. Roentgenol. 184(6), 1836–1842 (2005).
- Zalis ME, Perumpillichira JJ, Magee C, Kohlberg G, Hahn PF. Tagging-based, electronically cleansed CT colonography: evaluation of patient comfort and image readability. Radiology 239(1), 149–159 (2006).
- Campanella D, Morra L, Delsanto S et al. Comparison of three different iodine-based bowel regimens for CT colonography. Eur. Radiol. 20(2), 348–358 (2010).
- Liedenbaum MH, de Vries AH, van Rijn AF et al. CT colonography with limited bowel preparation for the detection of colorectal neoplasia in an FOBT positive screening population. Abdom. Imaging 35(6), 661–668 (2010).
- Fenlon HM, Nunes DP, Schroy PC 3rd, Barish MA, Clarke PD, Ferrucci JT. A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N. Engl. J. Med. 341, 1540–1542 (1999).
- Yee J, Akerkar GA, Hung RK, Steinauer- Gebauer AM, Wall SD, McQuaid KR. Colorectal neoplasia: performance characteristics of CT colonography for detection in 300 patients. Radiology 219, 685–692 (2001).
- Johnson CD, Harmsen WS, Wilson LA et al. Prospective blinded evaluation of computed tomographic colonography for screen detection of colorectal polyps. Gastroenterology 125, 311–319 (2003).
- Rockey DC, Paulson E, Niedzwiecki D et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 365, 305–311 (2005).
- Pickhardt PJ, Choi JR, Hwang I et al. CT virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N. Engl. J. Med. 349, 2191–2200 (2003).
- Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann. Intern. Med. 141, 352–359 (2004).
- Johnson CD, Chen MH, Toledano AY et al. Accuracy of CT colonography for detection of large adenomas and cancers. N. Engl. J. Med. 359, 1207–1217 (2008).
- Regge D, Laudi C, Galatola G et al. Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA 301, 2453–2461 (2009).
- Graser A, Steiber P, Nagel D et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy, and fecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut 58, 241–248 (2009).
- Cash BD, Kim C, Jensen D et al. Accuracy of computed tomographic colonography for colorectal cancer screening in asymptomatic individuals. Gastroenterology 132, A92 (2007).
- Pickhardt PJ, Hassan C, Halligan S, Marmo R. CT colonography and colonoscopy for detection – systematic review and meta-analysis. Radiology 259, 393–405 (2011).
- Fidler J, Johnson C, MacCarty R, Welch TJ, Hara AK, Harmsen WS. Detection of flat lesions in the colon with CT colonography. Abdom. Imaging 27, 292–300 (2002).
- Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: implications for screening with CT virtual colonoscopy. AJR Am. J. Roentgenol. 183, 1343–1347 (2004).
- Soetikno RM, Kaltenbach T, Rouse RV et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA 299, 1027–1035 (2008).
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest. Endosc. 58(Suppl. 6), S3–S43 (2003).
- Pickhardt PJ, Kim DH, Robbins JB. Flat (nonpolypoid) colorectal lesions identified at CT colonography in a U.S. screening population. Acad. Rad. 17, 784–790 (2010).
- Butterly LF, Chase MP, Pohl H, Fiarman GS. Prevalence of clinically important histology in small adenomas. Clin. Gastroenterol. Hepatol. 4, 343–348 (2006).
- Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 10, 1171–1178 (2009).
- Shah JP, Hynan LS, Rockey DC. Management of small polyps detected by screening CT colonography: patient and physician preferences. Am. J. Med. 122, 687.e1–9 (2009).
- Lieberman D, Moavec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology 135, 1100–1105 (2008).
- Berrington de Gonzalez A, Mahesh M, Kim KP et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch. Int. Med. 169, 2071–2077 (2009).
- Mettler FA, Bhargavan M, Faulkner K et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources – 1950–2007. Radiology 253, 520–531 (2009).
- Amis ES Jr, Butler PF, Applegate KE et al. American College of Radiology white paper on radiation dose in medicine. J. Am. Coll. Radiol. 4, 272–284 (2007).
- Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology 129, 328–337 (2005).
- Johnson KT, Johnson CD, Anderson SM, Bruesewitz MR, McCollough CH. CT colonography: determination of optimal CT technique using a novel colon phantom. Abdom. Imaging 29(2), 173–176 (2004).
- Wessling J, Fischbach R, Meier N et al. CT colonography: protocol optimization with multi-detector row CT – study in an anthropomorphic colon phantom. Radiology 228, 753–759 (2003).
- Iannaccone R, Laghi A, Catalano C et al. Detection of colorectal lesions: lower-dose multi-detector row helical CT colonography compared with conventional colonoscopy. Radiology 229, 775–781 (2003).
- van Gelder RE, Venema HW, Serlie IW et al. CT colonography at different radiation dose levels: feasibility of dose reduction. Radiology 224, 25–33 (2002).
- Macari M, Bini EJ, Xue X et al. Colorectal neoplasms: prospective comparison of thinsection low-dose multi-detector row CT colonography and conventional colonoscopy for detection. Radiology 224, 383–392 (2002).
- van Gelder RE, Venema HW, Florie J et al. CT colonography: feasibility of substantial dose reduction – comparison of medium to low doses in identical patients. Radiology 232, 611–620 (2004).
- Neri E, Vagli P, Turini F et al. Post-surgical follow-up of colorectal cancer: role of contrast-enhanced CT colonography. Abdom. Imaging 35, 669–675 (2010).
- Ferrucci J, Barish M. Virtual colonoscopy; letter to the editor. JAMA 292(4), 431 (2004).
- Friedman AC, Downing D, Chino J, Krupinski E, Kilian C, Lance P. Feasibility of remote CT colonography at two rural native American medical centers. AJR Am. J. Roentgenol. 195(5), 1110–1117 (2010).
- Young PE, Ray QP, Hwang I et al. Gastroenterologists’ interpretation of CTC: a pilot study demonstrating feasibility and similar accuracy compared with radiologists’ interpretation. Am. J. Gastroenterol. 104, 2926–2931 (2009).
- Hoppe H, Quattropani C, Spreng A, Mattich J, Netzer P, Dinkel HP. Virtual colon dissection with CT colonography compared with axial interpretation and conventional colonoscopy: preliminary results. AJR Am. J. Roentgenol. 182(5), 1151–1158 (2004).
- Yoshida H, Dachman A. CAD techniques, challenges, and controversies in computed tomographic colonography. Abdom. Imaging. 30(1), 26–41 (2005).
- Bielen D, Kiss G. Computer-aided detection for CT colonography: update 2007. Abdom. Imaging 32(5), 571–581 (2007).
- Park SH, Kim SY, Lee SS et al. Sensitivity of CT colonography for nonpolypoid colorectal lesions interpreted by human readers and with computer-aided detection. AJR Am. J. Roentgenol. 193, 70–78 (2009).
- Yoshida H, Masutani Y, MacEneaney P, Rubin DT, Dachman AH. Computerized detection of colonic polyps at CT colonography on the basis of volumetric features: pilot study. Radiology 222(2), 327–336 (2002).
- Yoshida H, Dachman AH. Computer-aided diagnosis for CT colonography. Semin. Ultrasound CT MR 25(5), 419–431 (2004).
- Yoshida H, Näppi J, MacEneaney P, Rubin DT, Dachman AH. Computer-aided diagnosis scheme for detection of polyps at CT colonography. Radiographics 22(4), 963–979 (2003).
- Yao J, Summers R. Employing topographical height map in colonic polyp measurement and false positive reduction. Pattern Recognit. 42, 1029–1040 (2009).
- Halligan S, Taylor SA, Dehmeshki J et al. Computer-assisted detection for CT colonography: external validation. Clin. Radiol. 61(9), 758–763 (2006).
- Hein PA, Krug LD, Romano VC, Kandel S, Hamm B, Rogalla P. Computer-aided detection in computed tomography colonography with full fecal tagging: comparison of standalone performance of 3 automated polyp detection systems. Assoc. Radiol. J. 61(2), 102–108 (2010).
- Kim DH, Pickhardt PJ, Hanson ME, Hinshaw JL. CT colonography: performance and program outcome measures in an older screening population. Radiology 254, 493–500 (2010).
- Zalis ME, Barish MA, Choi JR et al. CT colonography reporting and data system: a consensus proposal. Radiology 236, 3–9 (2005).
- Hara AK, Johnson CD, MacCarty RL, Welch TJ. Incidental extracolonic findings at CT colonography. Radiology 215, 353–357 (2000).
- Edwards JT, Wood CJ, Mendelson RM, Forbes GM. Extracolonic findings at virtual colonoscopy: implications for screening programs. Am. J. Gastroenterol. 96, 3009–3012 (2001).
- Gluecker TM, Johnson CD, Wilson LA et al. Extracolonic findings at CT colonography: evaluation of prevalence and cost in a screening population. Gastroenterology 124, 911–916 (2003).
- Yee J, Kumar NN, Godara S et al. Extracolonic abnormalities discovered incidentally at CT colonography in a male population. Radiology 236, 519–526 (2005).
- Macari M, Nevsky G, Bonavita J, Kim DC, Megibow AJ, Babb JS. CT colonography in senior vs nonsenior patients: extracolonic findings, recommendations for additional imaging and polyp prevalence. Radiology 259, 767–774 (2011).
- Pickhardt PJ, Hanson ME, Vanness DJ et al. Unsuspected extracolonic findings at screening CT colonography: clinical and economic impact. Radiology 249, 151–159 (2008).
- Pickhardt PJ, Kim DH, Meiners RJ et al. Colorectal and extracolonic cancers detected at screening CT colonography in 10,286 asymptomatic adults. Radiology 255(1), 83–88 (2010).
- Duncan J, Ugochukwu ON, Sweeney WB et al. Key features of an efficient colorectal cancer screening program based on CT colonography. Gastrointest. Endosc. 73(4), AB141 (2011).
- Schwartz DC, Dasher KJ, Said A et al. Impact of a CT colonography screening program on endoscopic colonoscopy in clinical practice. Am. J. Gastroenterol. 103, 346–351 (2008).
- Benson ME, Pier J, Kraft S et al. Impact of a CT colonography colorectal cancer screening program on optical colonoscopy: 5 year data. Gastrointest. Endosc. 71(5), AB129 (2010).
- Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology 129, 1151–1162 (2005).
- Kim DH, Pickhardt PJ, Taylor AJ et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N. Engl. J. Med. 357, 1403–1412 (2007).
- Knudsen AB, Lansdorp-Vogelaar I, Rutter CM et al. Cost–effectiveness of computed tomographic colonography screening for colorectal cancer in the medicare population. J. Natl Cancer Inst. 102(16), 1238–1252 (2010).
- Moawad FJ, Maydonovitch CL, Cullen PA, Barlow DS, Jensen DW, Cash BD. CT colonography may improve colorectal cancer screening compliance. AJR Am. J. Roentgenol. 195(5), 1118–1123 (2010).
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am. J. Gastroenterol. 104, 739–750 (2009).
- Howard K, Salkeld G, Pignone M et al. Preferences for CT colonography and colonoscopy as diagnostic tests for colorectal cancer: a discrete choice experiment. Value Health 14(8), 1146–1152 (2011).
- Ghanouni A, Smith SG, Halligan S et al. Public perceptions and preferences for CT colonography or colonoscopy in colorectal cancer screening. Patient Educ. Couns. 89(1), 116–121 (2012).
- Woolf SH. The best screening test for colorectal cancer – a personal choice. N. Engl. J. Med. 343, 1641–1643 (2000).
- Seeff LC, Manninen DL, Dong FB et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology 127(6), 1661–1669 (2004).
- Allison JE. Hepatology news. Promote ‘be screened’, not ‘get a colonoscopy’. GI Hepatology News 6(7), 14 (2012).
- Steinwachs D, Allen JD, Barlow WE et al. Enhancing use and quality of colorectal cancer screening. NIH Consens. Sci. Statements 27(1), 1–31 (2010).
- Blue Cross Blue Shield Association Technology Evaluation Center. CT colonography (‘virtual colonoscopy’) for colon cancer screening. Assessment Program 24(1), 1–15 (2009).
- Sarfaty M, Myers RE. The effect of HEDIS measurement of colorectal cancer screening on insurance plans in Pennsylvania. Am. J. Manag. Care 14(5), 277–282 (2008).
- Cash BD, Stamps K, McFarland EG, Spiegel AR, Wade SW. Clinical use of CT colonography for colorectal cancer screening in military training facilities and potential impact on HEDIS® measures. J. Am. Coll. Radiol. 10(1), 30–36 (2013).
- American Cancer Society: cancer facts and figures 2012. www.cancer.gov/cancertopics/types/ commoncancers (Accessed 4 December 2012)
- National Cancer Institute at the NIH. www.cancer.gov/cancertopics/types/colonand- rectal (Accessed 4 December 2012)
- American Cancer Society. Cancer prevention and early detection, facts and figures 2010. www.cdc.gov/cancer/colorectal/basic_info/ facts.htm (Accessed 5 December 2012)
- Release of the President’s medical exam. www.whitehouse.gov/the-press-office/ release-presidents-medical-exam (Accessed 29 December 2012)
• First major guideline to endorse CT colonography (CTC) as a colorectal cancer (CRC) screening test.
• Most recent US Preventive Services Task Force review of CRC screening modalities, which served as the basis for lack of Centers for Medicare and Medicaid Services coverage of CTC.
• • Multicenter study from diverse practices demonstrating preference for CTC compared with colonoscopy.
• Interesting study evaluating minimal bowel preparation for CTC.
• • First well-performed pivotal US trial showing equivalence of CTC to colonoscopy for detection of large polyps.
• • Comparison of CTC with multiple other modalities for CRC screening.
• • Study evaluating incremental gains in CRC screening compliance achievable with CTC.
■ Websites


