Review Article - Interventional Cardiology (2020) Volume 12, Issue 7
Zero and near-zero fluoroscopic ablation of cardiac arrhythmias: A review of green electrophysiology
- Corresponding Author:
- Zefeng Wang
Department of Cardiology,
Beijing Anzhen Hospital,
Capital Medical University,
Beijing, China,
E-mail:wangzefeng@139.com - Yongquan Wu
Department of Cardiology,
Beijing Anzhen Hospital,
Capital Medical University,
Beijing, China,
E-mail: wuyongquan67@163.com
Received date: October 25, 2020; Accepted date: November 16, 2020; Published date: November 23, 2020
Abstract
Radiofrequency catheter ablation is currently a first-line therapy for most cardiac tachyarrhythmias. Fluoroscopy harm for patients and medical staffs in the electrophysiology laboratory has for long-time been an issue of concern. For last decades, many measures have risen for zero and near-zero fluoroscopic ablation of cardiac arrythmias (green electrophysiology) including new Three-Dimensional Electro- Anatomical Mapping (3D-EAM) software, and Intracardiac Echocardiography (ICE) as most popular technologies. Recent years, some electrophysiologists innovated existing technologies and successfully achieved complete zero fluoroscopy, such as T3D (total three-dimension) and EAM-ICE, which make green electrophysiology more widely spread and used. However, fluoroscopy still plays a pivotal role in radiofrequency ablation in a wide range. Current methods have both advantages and disadvantages. The combination of existing technologies has pointed out a trend of green electrophysiology. All in all, the development prospect of green electrophysiology is bright in the future.
Keywords
Green electrophysiology • Zero fluoroscopy • Cardiac ablationV • Threedimensional electro-anatomical mapping • Intracardiac echocardiography
Introduction
Over the last two decades, the field of cardiac electrophysiology has undergone tremendous change and evolution. Radiofrequency catheter ablation has currently been a first-line therapy for most cardiac tachyarrhythmias. Fluoroscopy harm for patients and medical staffs in the electrophysiology laboratory has for long-time been an issue of concern. Therefore, many measures have risen for zero and near-zero fluoroscopic ablation of cardiac arrythmias (green electrophysiology) including new three-dimensional electro-anatomical mapping (3D-EAM) software, and Intracardiac Echocardiography (ICE) as most popular technologies. Recent years, some electrophysiologists innovated existing technologies and successfully achieved complete zero fluoroscopy, such as T3D (total three-dimension) and EAMICE, which make green electrophysiology more widely spread and used. In this article, we briefly reviewed the development and current status of green electrophysiology, and analyzed possible future development trends.
Overview
Radiofrequency Catheter Ablation (RFCA) is currently a first-line therapy for most supraventricular, atrioventricular and ventricular arrhythmias [1,2]. Fluoroscopy guidance has been playing an important role during the operation from the very beginning to the present [3-5]. Owing to the usually long duration of the operation, the radiation hazard to both patients and medical staffs in the electrophysiology laboratory is much concerned [6]. The use of fluoroscopy has been found associated with radiation-related increases in the lifetime risk of malignancies, genetic defects, skin injuries, and cataracts [6-11]. Additionally, orthopedic injuries may result from the use of heavy protective lead apparel [12]. Since there is no definite level of exposure known to be completely safe, the utilization of ionizing radiation during medical diagnostic or interventional procedures should be “as low as reasonably achievable” (ALARA principle) [13]. The good news is that various measures including the use of remote magnetic navigation system, new Three-Dimensional Electro-Anatomical Mapping (3D-EAM) software, Intracardiac Echocardiography (ICE), miniaturized Transesophageal Echocardiography (TEE), and contact-force sensing catheters, have led to a significant reduction or complete elimination of fluoroscopy in most patients, particularly in children, pregnant women, and patients with immune dysfunction [14-16]. All in all, “zero and near-zero” fluoroscopic ablation of cardiac arrhythmias, which is so called “green electrophysiology”, is gradually being introduced in clinical practice.
Throughout last two decades, green electrophysiology develops rapidly and now shows a certain trend [17-21]. There have been quite a few large trials (Table 1). Among the measures, remote magnetic navigation system rose earlier, but it failed to become a leader in green electrophysiology due to its expensive equipment and few supporting catheters. 3D-EAM, for its more flexible operating mode and shorter learning curve, has gradually become the most popular and widely-spread method. Recent years, Chinese electrophysiologists innovatively explored the 3D-EAM system, and summarized a complete set of zero fluoroscopic arrhythmia radiofrequency ablation technology named “T3D (total threedimension)”, which spread domestically and internationally rapidly. Another technology, Intracardiac Echocardiography (ICE), realizes complete zero fluoroscopic in real time. Some developed countries in Europe and America have adopted it as a routine. Nevertheless, the current green electrophysiology still needs the specialized equipment support of the electrophysiology center and long enough professional learning, and the promotion in primary hospitals is still a long process. In the future, the concept of green electrophysiology will be further popularized and promoted by more electrophysiologists. The integration of several existing technologies may be a better trend, and the emergence of newer technologies is just around the corner.
| Authors | Year | Country | Study type | Arrhythmia included | Method |
|---|---|---|---|---|---|
| Earley et al. [22] | 2006 | UK | pro RT | AVNRT, AVRT, AFL, Other | Ensite NavX, Carto |
| Smith et al. [23] | 2007 | Ohio | Re pro non-RT | AVNRT, WPW, concealed pathway | EnSite NavX |
| Alvarez et al. [15] | 2009 | Spain | Re pro non-RT | AVNRT | EnSite NavX |
| Sun et al. [24] | 2011 | China | pro RT | AVNRT, AVRT, AFL, other | EnSite NavX |
| Razminia et al. [25] | 2012 | USA | Re pro non-RT | AF, AFL, AT, AVNRT, AVRT, VT | EP-WorkMate, EnSite NavX, ICE |
| Stec et al. [26] | 2014 | Poland | pro non-RT | AVNRT, WPW/OAVRT, AFL, AT | |
| Mah et al. [20] | 2014 | USA | pro RT | AVNRT, WPW, Right AP, other | ICE + Carto 3, ICE |
| Bulava et al. [27] | 2015 | Czech Republic | pro RT | AF | ICE, Carto 3 |
| Casella et al. [28] | 2015 | Italy | pro RT | AVNRT, Right AP, Left AP, AFL, AT | EnSite NavX |
| Giaccardi et al. [19] | 2016 | Italy | Re pro non-RT | AVNRT, Right AFL, AP, AT, VT, other | EnSite NavX |
| Seizer et al. [29] | 2016 | Germany | Re pro non-RT | AVNRT, WPW, AT, Typical flutter | EnSite NavX |
| Luani et al. [30] | 2018 | Germany | pro non-RT | AVNRT | ICE |
| Pani et al. [31] | 2018 | Multicenter | pro non-RT | AVNRT, AP, AFL, AT | Carto 3 |
| Sommer et al. [32] | 2018 | Germany | pro non-RT | AF | MediGuide |
| Walsh et al. [33] | 2018 | Ireland | pro non-RT | AVNRT, AP, AT, AFL | Ensite Precision |
| Santoro et al. [34] | 2019 | Italy | pro non-RT | AVNRT, AVRT, focal AT, incisional AFL, idiopathic VT, PVC | Carto 3 |
| Luani et al. [35] | 2019 | Germany | pro non-RT | AVNRT | ICE |
| Chen et al. [36] | 2020 | China | pro non-RT | AVNRT, AVRT | Ensite NavX |
| Jan et al. [37] | 2020 | Holland | pro non-RT | AF | Ensite NavX/Carto + ICE |
Abbreviations: pro RT: Prospective Randomized Trial; pro non-RT: Prospective Nonrandomized Trial; Re pro non-RT: Retrospective Nonrandomized Trial; AVNRT: Atrioventricular Nodal Reentrant Tachycardia; WPW:WPW Syndrome; AVRT: Atrioventricular Reentrant Tachycardia; AFL :Atrial Flutter; AT: Atrial Tachycardia; AF: Atrial Fibrillation; AP: Atrial Pathway; VT: Ventricular Tachycardia; PVC: Premature Ventricular Contraction
Table 1: Trials covering mainstream green electrophysiological methods in last decades.
The Most Used Methods and the Situation in Clinical Practice
3D-EAM
3D-EAM systems, based on reconstruction model of the heart chambers, have emerged as an alternative with the potential to limit or avoid radiation exposure [19,31,38]. To date, four 3D-EAM systems are widely used to visualize electrophysiology catheters without X-rays: EnSite NavX and Mediguide technologies (both by St. Jude Medical, St Paul, MN, USA), Carto (Biosense Webster, Diamond Bar, CA, USA), and Rhythmia (Boston Scientific, San Jose, CA, USA).
The EnSite NavX relies on three pairs of nominally orthogonal skin patches in x-y and z-axis positioned on the patient’s chest, which create an electrical location field on the patient’s thorax. An additional abdomen patch serves as a reference during advancement of the catheters in the iliofemoral venous axis. The system collects electrical data from standard electrophysiology catheters to track or navigate their movement, construct 3D models of the chamber and create activation and voltage maps [39]. Later, the same company introduced Mediguide technology providing the possibility to move catheters into previously acquired fluoroscopic loops. Pre-acquired electrocardiogram and respiration triggered biplane short sequences of conventional fluoroscopic frames allow traditional catheter and structure visualization tracking within dynamic, virtual cardiac chamber models (4D model) [40]. Catheter positioning system is based on a dynamic electromagnetic field integrated with a miniaturized single coil sensor mounted on dedicated electrophysiology catheters and a reference sensor attached to the patient’s chest [41]. It should however be reminded that pre-acquired fluoroscopy loops only provide an illusion of real-time imaging. Acute clinical variations, such as a pericardial tamponade or a pneumothorax, will not be real-time depicted.
The Carto system, in its present third generation (Carto 3), instead, is based on six skin patches positioned on patient’s chest and back that create an electrical based location field. These patches, by a location pad technology (with nine coils), also create a magnetic field. The combination is an electromagnetic field in which catheter movements can be detected. As the catheter moves around the chamber, a multitude of such associated locations are created and stored by the system. Advanced Catheter Location Technology combines the magnetic location technology with current based visualization data in a virtual chamber reference system built by catheter movements. This technology offers the possibility of merging the virtual chamber with a pre-acquired anatomical image [e.g. magnetic resonance imaging (MRI) or computed tomography] allowing physicians to navigate catheters in an accurate representation of the patient’s anatomy. Additionally, with this system, activation mapping information, during arrhythmias or sinus rhythm, may be projected to the map with a color-coded mode, useful for guiding the ablation to the origin of the arrhythmia. However, due to the magnetic field, it is limited to track the catheter in vessels between the access point and the heart. In cases of catheter torsion, impaction, kinking, vessel branches, stenosis, and malformations, tracking the catheter in the vessels is essential to enable the CARTO system to cover vessels with magnetic fields and track the catheters inside the vessels without moving the ablation catheter tip to the desired part [42]. The Carto-Univu, a module permitting real-time catheter tracking superimposed on pre-recorded cine loops, has further implemented the potential of this system. Recently a new 3D-EAM, Rhythmia, based on both magnetic and impedance 1-2 mm accuracy localization, has been introduced. This system is based on an open architecture permitting the choice of different diagnostic catheters. However, activation and substrate mapping can be performed only with a dedicated catheter (IntellaMap OrionTM) that has the peculiarity of being a basket, high-resolution mapping catheter with 64 low-noise electrodes and 2.5 mm inter-electrode spacing. Thanks to the latter and an advanced point acquisition software and process, this system is able to generate, by automated and continuous mapping, accurate, high-resolution 3D-EAMs.
The recently implemented AcQMap system [43], combining ultrasound guided anatomical details to high density bipolar or unipolar voltage signals obtained from a hybrid catheter with 48 ultrasound transducers and 48 electrodes, seems indeed promising. Eventually, having cardiac MRI the capability to show both anatomic and functional tissue data without ionizing radiation, growing interest is directed towards the possibility of real-time MRI catheter tracking, obviously, in this case, not only in the electrophysiological field [44].
Though both have similar effectiveness and safety and both reduce fluoroscopy, NavX has a significantly greater effect than CARTO, especially for atrioventricular nodal reentrant tachycardia (AVNRT) [22].
Yu et al. took the lead in carrying out the first full three-dimensional and full-course zero-ray interventional operation without radiation and ultrasound in the world. Subsequently, the technology was applied to the radiofrequency ablation treatment of arrhythmia in patients of all ages. Its safety and effectiveness are consistent with conventional atrial fibrillation ablation, which has also been verified in elderly patients with paroxysmal atrial fibrillation.
Intracardiac echocardiography (ICE)
Intracardiac echocardiography (ICE) has emerged as a major development in real-time cardiac imaging and is an integral to the safety and efficacy of electrophysiological procedures as a complementary modality to fluoroscopy for transseptal puncture [45]. ICE has the potential to recognize complications early and improve efficacy of the procedure by providing simultaneous anatomical correlation to intracardiac signals [20,30,35,46-50].
In atrial fibrillation (AF) ablation, the utility of ICE has been most best-demonstrated during transseptal access into the left atrium [51]. It provides direct visualization of the positioning of the transseptal needle which facilitates avoidance of critical anatomical structures. Additionally, it aids in the recognition of anatomical variations in the anatomy of the pulmonary veins [52] and can direct the safe positioning of the ablation catheter for effective delivery of radiofrequency lesions [53]. Near instantaneous identification of potential complications, such as a pericardial effusion if the patient becomes hypotensive or formation of a thrombus on the catheter tip when the impedance is seen to rise during ablation, is also possible [51]. The use of ICE during AF ablation has further been shown to decrease the recurrence of AF and potentially improve long-term outcomes.
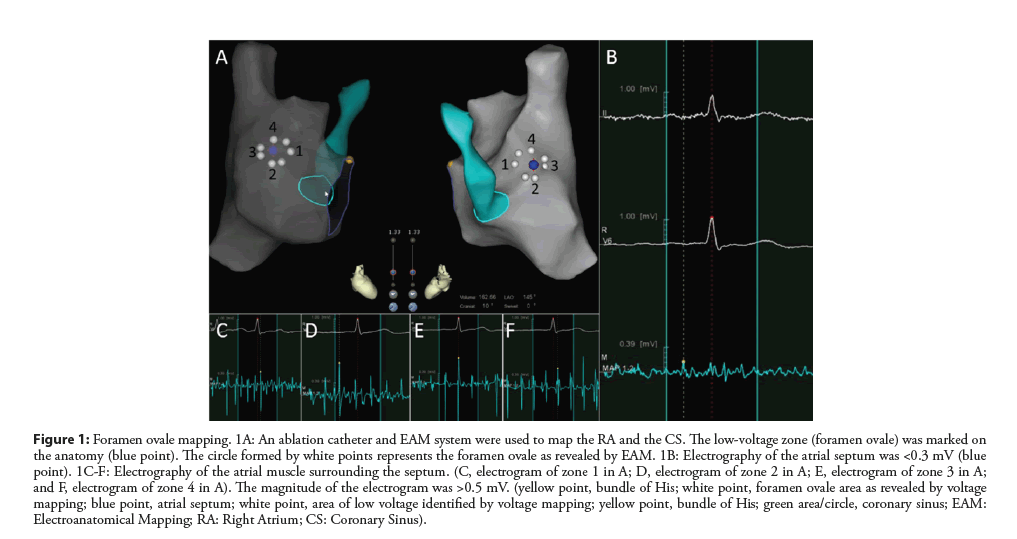
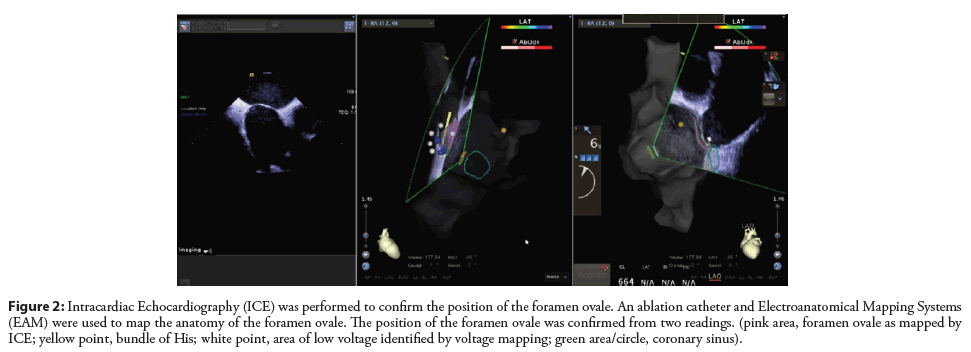
Zhang et al. combined right atrial electroanatomical mapping with SoundStar 3D diagnostic ultrasound catheter (EAMICE) and evaluated the efficiency and safety of zero-fluoroscopy transseptal puncture [54]. In their study, a low-voltage area was mapped in the area of the atrial septum after direct RA mapping using EAM system. This low-voltage area is an optimal location for transseptal puncture without the use of fluoroscopy (Figure 1). The ICE probe was then placed inside the RA using an 11-F introducer. The position of the atrial septum was confirmed by ICE (Figure 2). EAM-ICE upgrades the function of ICE by providing 3D visualization of anatomical structures, thus making the accompanying procedure more effective and safer.
Figure 1: Foramen ovale mapping. 1A: An ablation catheter and EAM system were used to map the RA and the CS. The low-voltage zone (foramen ovale) was marked on the anatomy (blue point). The circle formed by white points represents the foramen ovale as revealed by EAM. 1B: Electrography of the atrial septum was <0.3 mV (blue point). 1C-F: Electrography of the atrial muscle surrounding the septum. (C, electrogram of zone 1 in A; D, electrogram of zone 2 in A; E, electrogram of zone 3 in A; and F, electrogram of zone 4 in A). The magnitude of the electrogram was >0.5 mV. (yellow point, bundle of His; white point, foramen ovale area as revealed by voltage mapping; blue point, atrial septum; white point, area of low voltage identified by voltage mapping; yellow point, bundle of His; green area/circle, coronary sinus; EAM: Electroanatomical Mapping; RA: Right Atrium; CS: Coronary Sinus).
Figure 2: Intracardiac Echocardiography (ICE) was performed to confirm the position of the foramen ovale. An ablation catheter and Electroanatomical Mapping Systems (EAM) were used to map the anatomy of the foramen ovale. The position of the foramen ovale was confirmed from two readings. (pink area, foramen ovale as mapped by ICE; yellow point, bundle of His; white point, area of low voltage identified by voltage mapping; green area/circle, coronary sinus).
Discussion
Green electrophysiology for supraventricular tachycardia: Where we are now?
The past decades have witnessed the great revolution of green electrophysiology, as reported by single [18,20,23,55-58], multicenter [19,31,38] experiences, metaanalysis [59,60] and randomize trial [28]. More than 50% of procedure were concluded without fluoroscopy (zero fluoroscopy), while in some cases fluoroscopy was necessary for additional maneuvers, but with a final radiological dose consistently lower (near-zero fluoroscopy) [57]. The clear evidence emerging is that 3D-EAM systems broadly reduced fluoroscopy exposure without affecting procedure safety and outcome for tachycardia such as typical atrial flutter, atrioventricular nodal re-entrant tachycardia, atrioventricular reentrant tachycardia and ventricular tachycardia [21]. A prospective, multicenter, randomized controlled trial (NO-PARTY trial) was designed to compare a minimally fluoroscopic RFCA guided by the EnSite NavX system with conventional fluoroscopyguided RFCA for supraventricular tachycardia (SVT) in terms of ionizing radiation exposure for both patient and operator and to estimate patients’ lifetime attributable risks associated with such exposure [28]. It reported that the use of a minimally fluoroscopic approach (MFA) with the EnSite NavX system is associated with a significant reduction in total fluoroscopy time, patients’ exposure, and operator radiation dose without any significant difference in terms of success and complication rates. The reduction in patients’ exposure shows a 96% reduction in the estimated risks of cancer incidence and mortality and an important reduction in estimated years of life lost and years of life affected. The increase in life expectancy and in the period of cancer-free life makes the MFA economically affordable at a rough economical analysis. Thus, faced with such an advantage in terms of cancer prevention, it would be desirable that MFA procedures were increasingly performed, at least in young patients. Fadhle et al. [42] found no significant difference in terms of the efficiency and safety for ablation of SVT comparing using CARTO system with the EnSite system or a conventional fluoroscopic approach. The T3D method based on CARTO 3 promoted by Chinese electrophysiologists combining the results of the preoperative imaging examination with the intraoperative 3D-EAM system, the true intraoperative zero rays are realized. However, it requires a good comprehension of anatomical knowledge and takes time to get familiar with this process.
Atrial fibrillation (AF): Successful combination of existing methods
AF is the most common arrhythmia in the adult population (particularly in older patients), and it represents the widest indication for catheter ablation. Fluoroscopic-based catheter ablation has been traditionally used to define anatomical landmarks, visualize catheters, and guide transseptal puncture [61]. Ever since 2009 Ferguson et al. first reported the result of their study of catheter ablation of AF using ICE and EAM with no fluoroscopy for the entire case, many researchers have showed successful zerofluoroscopic approach in RFCA of AF. 3D-EAM systems able to evaluate instantaneous catheter positioning, respiration triggered movement and offering the possibility of integrating radiological images are allowing users to perform pulmonary vein isolation and left atrial substrate modification with minimal use of fluoroscopy [46,62,63]. In addition to 3D-EAM systems, other technologies have emerged to facilitate AF ablation: contact force technology, for example, is able to monitor and measure the tissue/catheter contact in order to avoid excessive or insufficient forces on the tip of the catheter. After a brief learning curve, this technology further supports manoeuvring in a zero fluoroscopy setting [29,64]. More recently, a prospective, randomized, blinded trial, clearly showed that the systematic use of third-generation EAM systems reduced fluoroscopy exposure in patients undergoing AF ablation, without increasing procedure duration or affecting safety and short-term efficacy [65]. Eventually, the systematic use of EAM systems integrated with preacquired imaging, in this case cardiac magnetic resonance with use of oral gadobenate dimeglumine [66], also presents the advantage of visualizing the esophagus, potentially limiting occurrence of atrio-oesophageal fistulas, a rare but potentially fatal periprocedural complication. As previously stated, to date, the only remaining phase that limits a complete zero fluoroscopy approach for AF ablation, and other left-sided arrhythmias not approachable by retrograde aortic access, is transeptal puncture (TS). In this respect, preliminary data suggest that ICE may become a routine strategy to guide TS without fluoroscopy use [63,66]. Using a third-generation mapping system, contact force technology and ICE guiding TS, RF ablation of AF was not only feasible without fluoroscopy but also safe, without affecting procedure duration, radiofrequency application time and mid-term efficacy [27]. Mansour et al [67]. Recently reported on the use of the equipment compatible with the Mediguide Technology in order to perform a TS without fluoroscopy in a small population of consecutive patients. In fact, by the use of a guidewire with a magnetic sensor on the tip, the authors managed to perform the TS with a very low fluoroscopy exposure.
Problems existing and potential solution
At present, zero and near-zero fluoroscopy approach still has some limitations. Of 3D-EAM system, the heart configuration is collected point by point through the catheter, which is a virtual space projection and somewhat different from the real anatomy. Without sufficient understanding of the special anatomy of the cardiac ablation site, the operation risk may be increased. Thus, a learning curve is needed [68], but can be fulfilled quickly, as most electrophysiologists are already using 3D-EAM in ablation procedures. A shorter learning curve can be anticipated with adult patients [69]. Besides, 3D-EAM is not applicable to patients with arrhythmias arising from the aortic cusps, with newly implanted intracardiac leads, and with epicardial arrhythmias [25]. The position of important anatomical structures, such as the His bundle, should be rechecked if the ablation site is in a high-risk area. Nonetheless, the accuracy of the geometries generated by 3D-EAM systems depends on physical and technical parameters, such as the stability of the reference catheter, intracardiac or thoracic impedance, electromagnetic interference in the EP laboratory, etc. Unrecognized change of these parameters during the procedure could cause a geometry drift, which might have deleterious effects when ablating in a relatively small anatomical area such as the triangle of Koch (in close vicinity of the compact AV node or fast pathway). In this context, the collision of the ablation catheter with the reference one could cause a dislocation of the latter leading to an inevitable geometry drift, which would require a careful correction of the reference catheter and might discourage the operator from avoiding fluoroscopic catheter guidance. With Ensite respiration, compensation should be repeated when the patient exhibits apparent changes in respiratory amplitude. Additional electrodes make it a little more costly. While Casella et al. [28] conformed that the increase in life expectancy and in period of life without cancer made minimally fluoroscopic approach economically affordable at a rough economical analysis. The cost may be cut down in the future through more widespread use of this technology.
Some researchers found 3D-EAMs associated with longer procedure times as compared to fluoroscopy guidance [70,71]. However, Razminia et al. demonstrated fluoroless ablation did not compromise procedural time in their 5 years of experience [72].
ICE still requires a dedicated operator and an additional venous puncture, potentially increasing the risk of vascular complications, which can result in longer ablation procedures and may increase the cost of the procedure. There are also challenges in patients with preexisting pacemaker and defibrillator leads, those with atrial septal defect closure devices, and those with anatomic anomalies or poor acoustic windows. Short flashes if fluoroscopy may be appropriate. Moreover, using of ICE makes greater expense for patients.
Outlook
With the position of radiofrequency ablation in the treatment of arrhythmia gradually consolidated, there is no doubt about the clinical application and the promotion of green electrophysiology. Advanced equipment and experienced electrophysiologists are equally important. In a word, the future of green electrophysiology is bright. It is expected that the staffs can get rid of the heavy lead clothes and the support of large equipment.
Conclusion
With Green electrophysiology is developing rapidly at present. Although many electrophysiologists are proficient in using various techniques, fluoroscopy still plays a pivotal role in the positioning of anatomic examination and ablation site and complication prevention of radiofrequency ablation in a wide range. Cost and efficiency are issues that cannot be ignored, and shortening the learning curve is also the unremitting pursuit of the majority of electrophysiologists. The combination of existing technologies has pointed out a way.
References
- Josep B, Katritsis DG, Elena A, et al. 2019: ESC Guidelines for the management of patients with supraventricular tachycardia The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J 41(5): 655–720 (2020).
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017: AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Hea. Circulation. (2018).
- Picano E, Vano E. The Radiation Issue in Cardiology: The time for action is now. Cardiovasc Ultrasound. 9(1): 35 (2011).
- Vañó E, González L, Guibelalde E, et al. Radiation exposure to medical staff in interventional and cardiac radiology. Br J Radiol. 71(849): 954 (1998).
- Estner HL, Maria GB, Jian C, et al. Use of fluoroscopy in clinical electrophysiology in Europe: Results of the European Heart Rhythm Association Survey. Europace. 17(7): 1578–1583 (2015).
- Vañó E, Arranz L, Sastre JM, et al. Dosimetric and radiation protection considerations based on some cases of patient skin injuries in interventional cardiology. Br J Radiol. 71(845): 510–516 (1998).
- Kovoor P, Ricciardello M, Collins L, et al. Risk to Patients from Radiation Associated With Radiofrequency Ablation for Supraventricular Tachycardia. Circulation. 98(15): 1534–1540 (1998).
- Calkins H, Niklason L, Sousa J, et al. Radiation exposure during radiofrequency catheter ablation of accessory atrioventricular connections. Circulation. 84(6): 2376–2382 (1991).
- Rehani MM, Ortiz-Lopez P. Radiation effects in fluoroscopically guided cardiac interventions--keeping them under control. Int. J. Cardiol. 109(2): 147–151 (2006).
- Park TH, Eichling JO, Schechtman KB, et al. Risk of Radiation Induced Skin Injuries from Arrhythmia Ablation Procedures. Pacing Clin Electrophysiol. 19(9): 1363–1369 (2010).
- Mcfadden SL, Mooney RB, Shepherd PH. X-ray dose and associated risks from radiofrequency catheter ablation procedures. Br J Radiol. 75(891): 253–265 (2002).
- Klein LW, Miller DL, Balter S, et al. Occupational Health Hazards in the Interventional Laboratory: Time for a Safer Environment. Radiology. 250(2): 538-544 (2009).
- Limacher MC, Douglas PS, Germano G, et al. ACC expert consensus document. Radiation safety in the practice of cardiology. American College of Cardiology. J Am Coll Cardiol. 31(4): 892–913 (1998).
- Germanas M, Grazia BM, Nikolaos D, et al. X-ray exposure hazards for physicians performing ablation procedures and device implantation: Results of the European Heart Rhythm Association survey. Europace. 15(3): 444–446 (2013).
- Alvarez M, Tercedor L, Almansa I, et al. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm. 6(12): 1714–1720 (2009).
- Wan G, Shannon KM, Moore JP. Factors associated with fluoroscopy exposure during pediatric catheter ablation utilizing electroanatomical mapping. J Interv Card Electrophysiol. 35(2): 235–242 (2012).
- Macías R, Uribe I, Tercedor L, et al. A zero-fluoroscopy approach to cavotricuspid isthmus catheter ablation: Comparative analysis of two electroanatomical mapping systems. Pacing Clin Electrophysiol. 37(8): 1029-37 (2014).
- Casella M, Pelargonio G, Dello RA, et al. “Near-zero” fluoroscopic exposure in supraventricular arrhythmia ablation using the EnSite NavXTM mapping system: Personal experience and review of the literature. J. Interv. Card. Electrophysiol. 31(2): 109-118 (2011).
- Giaccardi M, Del Rosso A, Guarnaccia V, et al. Near-zero x-ray in arrhythmia ablation using a 3-dimensional electroanatomic mapping system: A multicenter experience. Heart Rhythm. 13(1): 150–156 (2016).
- Mah DY, Miyake CY, Sherwin ED, et al. The use of an integrated electroanatomic mapping system and intracardiac echocardiography to reduce radiation exposure in children and young adults undergoing ablation of supraventricular tachycardia. Europace. 16(2): 277–283 (2014).
- Fiorenzo G, Guerra PG, Alberto B, et al. The dream of near-zero X-rays ablation comes true. Eur Heart J. 37(36): 2749-2755 (2016).
- Earley MJ, Refai S, Maysaa A, et al. Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: A prospective randomized trial. Eur Heart J. 27(10): 1223 (2006).
- Smith G, Clark JM. Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing Clin Electrophysiol. 30(4): 510–518 (2010).
- Sun X, Xu J, Su H, et al. Near-zero exposure radiofrequency ablation of paroxysmal supraventricular tachycardia guided by EnSite NavX mapping. Scientific Res essays. 6(24): 5253–5260 (2011).
- Razminia M, Manankil MF, Eryazici PLS, et al. Nonfluoroscopic Catheter Ablation of Cardiac Arrhythmias in Adults: Feasibility, Safety, and Efficacy. J Cardiovasc Electrophysiol. 23(10): 1078-86 (2012).
- Stec S, Śledź J, Mazij M, et al. Feasibility of implementation of a “simplified, No-X-Ray, no-lead apron, two-catheter approach” for ablation of supraventricular arrhythmias in children and adults. J Cardiovasc Electrophysiol. 25(8): 866-874 (2014).
- Bulava A, Hanis J, Eisenberger M. Catheter Ablation of Atrial Fibrillation Using Zero‐Fluoroscopy Technique: A Randomized Trial. Pacing Clin Electrophysiol. 38(7): 797–806 (2015).
- Michela C, Antonio DR, Gemma P, et al. Near zero fluoroscopic exposure during catheter ablation of supraventricular arrhythmias: The NO-PARTY multicentre randomized trial. Europace. 18(10): 1565–1572 (2016).
- Kerst G, Weig HJ, Weretka S, et al. Contact force-controlled zero-fluoroscopy catheter ablation of right-sided and left atrial arrhythmia substrates. Hear Rhythm Off J Hear Rhythm Soc. 9(5): 709–714 (2012).
- Luani B, Zrenner B, Basho M, et al. Zero‐fluoroscopy cryothermal ablation of atrioventricular nodal re‐entry tachycardia guided by endovascular and endocardial catheter visualization using intracardiac echocardiography (Ice&ICE Trial). J Cardiovasc Electrophysiol. 29(1): 160-66 (2018).
- Pani A, Giuseppina B, Bonanno C, et al. Predictors of Zero X-Ray Ablation for Supraventricular Tachycardias in a Nationwide Multicenter Experience. Circ Arrhythmia Electrophysiol. 11(3): e005592 (2018).
- Sommer P, Bertagnolli L, Kircher S, et al. Safety profile of near-zero fluoroscopy atrial fibrillation ablation with non-fluoroscopic catheter visualization: Experience from 1000 consecutive procedures. Europace. (2018).
- Walsh KA, Galvin J, Keaney J, et al. First experience with zero-fluoroscopic ablation for supraventricular tachycardias using a novel impedance and magnetic-field-based mapping system. Clin Res Cardiol. 20(12): 1952-1958 (2018).
- Santoro A, Di Clemente F, Baiocchi C, et al. From near-zero to zero fluoroscopy catheter ablation procedures. J Cardiovasc Electrophysiol. 30(11): 2397-2404 (2019).
- Luani B, Rauwolf T, Genz C, et al. Intracardiac echocardiography versus fluoroscopy for endovascular and endocardial catheter navigation during cryo-ablation of the slow pathway in AVNRT patients. Cardiovasc. Ultrasound. 17(1): 12 (2019).
- Chen G, Wang Y, Proietti R, et al. Zero-fluoroscopy approach for ablation of supraventricular tachycardia using the Ensite NavX system: A multicenter experience. BMC Cardiovasc Disord. 20(1): 48 (2020).
- Jan M, Žižek D, Kuhelj D, et al. Combined use of electro-anatomic mapping system and intracardiac echocardiography to achieve zero-fluoroscopy catheter ablation for treatment of paroxysmal atrial fibrillation: a single centre experience. Int J Cardiovasc Imaging. 36(3): 415-422 (2020).
- Marzia G, Giuseppe M, Alessandro PP, et al. Long-term outcomes after “Zero X-ray” arrhythmia ablation. J Interv Card Electrophysiol. 54(1): 43-48 (2018).
- Borlich M, Sommer P. Cardiac Mapping Systems: Rhythmia, Topera, EnSite Precision, and CARTO. Card Electrophysiol Clin. 11(3): 449-458 (2019).
- Fenici R, Brisinda D. From 3D to 4D imaging: Is that useful for interventional cardiac electrophysiology? Conf Proc IEEE Eng Med Biol Soc. 2007: 5996–5999 (2007).
- Rolf S, Sommer P, Gaspar T, et al. Ablation of atrial fibrillation using novel 4-dimensional catheter tracking within autoregistered left atrial angiograms. Circ Arrhythmia Electrophysiol. 5(4): 684–690 (2012).
- Fadhle A, Hu M, Wang Y. The safety and efficacy of zero-fluoroscopy ablation versus conventional ablation in patients with supraventricular tachycardia. Kardiol Pol. 78(6): 552-558 (2020).
- Willems S. Mechanistic mapping of cardiac arrhythmias. Eur Heart J. 36(39): 2628 (2015).
- Elbes D, Magat J, Govari A, et al. Magnetic resonance imaging-compatible circular mapping catheter: An in vivo feasibility and safety study. Europace. 19(3): 458-464 (2016).
- Isath A, Padmanabhan D, Haider SW, et al. Does the use of intracardiac echocardiography during atrial fibrillation catheter ablation improve outcomes and cost? A nationwide 14-year analysis from 2001 to 2014. J Interv Card Electrophysiol. (2020).
- Ferguson JD, Helms A, Mangrum JM, et al. Catheter Ablation of Atrial Fibrillation Without Fluoroscopy Using Intracardiac Echocardiography and Electroanatomic Mapping Circ Arrhythmia Electrophysiol. 2(6): 611–619 (2009).
- Jan M, Kalinek TP, Tublar J, et al. Intra-cardiac ultrasound guided approach for catheter ablation of typical right free wall accessory pathways. BMC Cardiovasc Disord. 20(1): 210 (2020).
- Luca R, Diego P, Quinto VG. Intracardiac echocardiography catheter-guided zero fluoroscopy transeptal puncture technique for ablation of left-sided accessory pathway in a pregnant woman. Europace. 19(11): 1825 (2017).
- Jan M, Žižek D, Kuhelj D, et al. Combined use of electro-anatomic mapping system and intracardiac echocardiography to achieve zero-fluoroscopy catheter ablation for treatment of paroxysmal atrial fibrillation: A single centre experience. 36(3): 415-422 (2020).
- Iek D, Antoli B, Kalinek TP, et al. Intracardiac echocardiography-guided transseptal puncture for fluoroless catheter ablation of left-sided tachycardias. J Interv Card Electrophysiol. (2020).
- Ren JF. Practical Intracardiac Echocardiography in Electrophysiology. (2020).
- Szili-Torok T, McFadden EP, Jordaens LJ, et al. Visualization of elusive structures using intracardiac echocardiography: insights from electrophysiology. Cardiovasc Ultrasound. 2: 6 (2004).
- Kalman JM, Fitzpatrick AP, Olgin JE, et al. Biophysical characteristics of radiofrequency lesion formation in vivo: Dynamics of catheter tip-tissue contact evaluated by intracardiac echocardiography. Am Heart J. 133(1): 0–18 (1997).
- Zhang G, Cheng L, Liang Z, et al. Zero-fluoroscopy transseptal puncture guided by right atrial electroanatomical mapping combined with intracardiac echocardiography: A single-center experience. Clin Cardiol. 43(9): 1009-1016 (2020).
- Triedman JK, Miyake CY, Mah DY, et al. Nonfluoroscopic imaging systems reduce radiation exposure in children undergoing ablation of supraventricular tachycardia. Hear Rhythm Off J Hear Rhythm Soc. 8(4): 519–525 (2011).
- Marian C, Carsten W, Stefanie M, et al. Fluoroscopy integrated 3D mapping significantly reduces radiation exposure during ablation for a wide spectrum of cardiac arrhythmias. Europace. 17(6): 928-37 (2015).
- Cori A Di, Zucchelli G, Segreti L, et al. Predictors of zero X ray procedures in supraventricular arrhythmias ablation. Int J Cardiovasc Imaging. 36(9): 1599-1607 (2020).
- Haegeli LM, Stutz L, Mohsen M, et al. Feasibility of zero or near zero fluoroscopy during catheter ablation procedures. Cardiol J. 26(3): 226-232 (2019).
- Shurrab M, Laish-Farkash A, Lashevsky I, et al. Three-dimensional localization versus fluoroscopically only guided ablations: a meta-analysis. Scand Cardiovasc J. 47(4): 200–209 (2013).
- Yang L, Sun G, Chen X, et al. Meta-Analysis of Zero or Near-Zero Fluoroscopy Use During Ablation of Cardiac Arrhythmias. Am J Cardiol. 118(10): 1511-1518 (2016).
- Kochar A, Ahmed T, Donnellan E, et al. Operator learning curve and clinical outcomes of zero fluoroscopy catheter ablation of atrial fibrillation, supraventricular tachycardia, and ventricular arrhythmias. J Interv Card Electrophysiol. (2020).
- Scaglione M, Biasco L, Caponi D, et al. Visualization of multiple catheters with electroanatomical mapping reduces X-ray exposure during atrial fibrillation ablation. Europace. 13(7): 955 (2011).
- Ponti R De. Reduction of radiation exposure in catheter ablation of atrial fibrillation: Lesson learned. World J Cardiol. 7(8): 442 (2015).
- Giuseppe S, Francesco S, Leonardo C, et al. Catheter–tissue contact force for pulmonary veins isolation: a pilot multicentre study on effect on procedure and fluoroscopy time. Europace. 16(3): 335–340 (2014).
- Huo Y, Christoph M, Forkmann M, et al. Reduction of radiation exposure during atrial fibrillation ablation using a novel fluoroscopy image integrated 3-dimensional electroanatomic mapping system: A prospective, randomized, single-blind, and controlled study. Heart Rhythm. 12(9): 1945–1955 (2015).
- Faletti R, Rapellino A, Barisone F, et al. Use of oral gadobenate dimeglumine to visualise the oesophagus during magnetic resonance angiography in patients with atrial fibrillation prior to catheter ablation. J Cardiovasc Magn Reson. 16(1): 41 (2014).
- Mansour M, Afzal MR, Gunda S, et al. Feasibility of Transseptal Puncture Using a Nonfluoroscopic Catheter Tracking System. Pacing Clin Electrophysiol Pace. 38(7): 791–796 (2015).
- Gist K, Tigges C, Smith G, et al. Learning curve for zero-fluoroscopy catheter ablation of AVNRT: Early versus late experience. Pacing Clin Electrophysiol Pace. 34(3): 264–268 (2015).
- Tuzcu V. A Nonfluoroscopic Approach for Electrophysiology and Catheter Ablation Procedures Using a Three-Dimensional Navigation System. Pacing Clin Electrophysiol. 30(4): 519–525 (2010).
- Scaglione M, Ebrille E, Caponi D, et al. Single Center Experience of Fluoroless AVNRT Ablation Guided by Electroanatomic Reconstruction in Children and Adolescents. Pacing Clin Electrophysiol. 36(12): 1460–1467 (2013).
- Clark BC, Sumihara K, Mccarter R, et al. Getting to zero: Impact of electroanatomical mapping on fluoroscopy use in pediatric catheter ablation. J. Interv. Card. Electrophysiol. 46(2): 183–189 (2016).
- Razminia M, Willoughby MC, Demo H, et al. Fluoroless Catheter Ablation of Cardiac Arrhythmias: A 5-Year Experience. Pacing Clin Electrophysiol. 40(4): 425-433 (2017).