Research Article - Interventional Cardiology (2022) Volume 14, Issue 3
Zero-fluoroscopy catheter ablation for right ventricular outflow tract ventricular arrhythmias with a novel initial approach
- Corresponding Author:
- Huu Cong Nguyen
Cardiovascular Center, E Hospital, Hanoi, Vietnam,
E-mail: bacsyhuu@trungtamtimmach.vn
Received date: 19-Apr-2022, Manuscript No. FMIC-22-61192; Editor assigned: 21-Apr -2022, PreQC No. FMIC-22-61192 (PQ); Reviewed date: 09-May-2022, QC No. FMIC-22-61192; Revised date: 16-May-2022, Manuscript No. FMIC-22-61192 (R); Published date: 23-May-2022, DOI: 10.37532/1755-5310.2022.14(3).519
Abstract
Background: Radio Frequency Catheter Ablation (RFCA) for Right Ventricular Outflow Tract Ventricular Arrhythmias (RVOT VAs) without fluoroscopy is increasingly performed but usually uses steerable catheter and intracardiac echocardiography, all of which may be too expensive or unavailable in many places. We introduced a novel approach which uses a non-steerable decapolar catheter to map the RVOT first without the assistance of echocardiography.
Methods and findings: A single center, prospective study in patients undergoing catheter ablation for RVOT VAs from May 2020 to November 2021. The approach includes first using a non-steerable decapolar catheter to fluorelessly map the RVOT via the superior vena cava, then the ablation catheter is advanced to the RVOT via the femoral vein followed the 3D-road map built by quadripolar catheter. A total of 43 consecutive patients with electrocardiographic features of RVOT VAs were enrolled. Mean age was 53.5 (23-83 years old), female/male rate was 3/1. Most of the patients presented with only PVC (79%), while 09 patients (20.9%) with non-sustained VTs, and only 01 patient with sustained VT. In 38 patients (88.4%) with total-zero fluoroscopy, acute success rate was 100% without any significant complication. In the rest 5 cases who required minimal fluoroscopy, acute success rate was 80%, the procedure failed in 1 patient due to the epicardial site of the VA foci. After a mean follow-up time of 4.8 ± 5.1 months, the long-term success rates of fluoroless ablation and combined ablation were 89.5% and 75%, respectively. Overall, the acute success rate reached 97.7%, the long-term success rate was 86.0%, and the recurrence rate was 11.6%.
Conclusion: Performing fluoroless RVOT approach using further non-steerable decapolar diagnostic catheter via SVC without echocardiography is feasible and safe. The non-steerable decapolar catheter could be used for activation mapping of RVOT VAs as an initial strategy of procedure.
Keywords
Right ventricular outflow tract arrhythmias • Non-steerable diagnostic catheter • Zero-fluoroscopy approach
Introduction
Radio Frequency Catheter Ablation (RFCA) for Right Ventricular Outflow Track (RVOT) Ventricular Arrhythmias (VAs) is normally performed under fluoroscopy guidance. However, some studies have reported the risks of radiation exposure for patients and catheterization lab personnel, especially for complex/ lengthy procedure or pediatric, obese, and pregnant patients [1,2]. Therefore, it is desirable to reduce radiation exposure in electrophysiology procedure, specifically, to keep radiation dose “as low as reasonably achievable” [3]. The advent of three dimensional (3D) electroanatomical mapping and intracardiac echocardiography (ICE) has allowed operators to significantly reduce radiation exposure [4], even to achieve zero-fluoroscopy level for idiopathic ventricular arrhythmias and is increasingly used with demonstrated safety and efficacy by some authors [5-7].
For fluoroless catheter ablation of idiopathic RVOT VAs, the initial strategy for mapping is conventionally to use a deflectable catheter such as a steerable mapping catheter or an ablation catheter through femoral vein and Inferior Vena Cava (IVC) [5- 11]. Some authors have added Intracardiac Echocardiography (ICE) system to aid in approaching the RVOT [7,12]. Due to the unavailability of ICE system and steerable mapping catheter in Viet Nam, we initially had to use the bidirectional ablation catheter via the IVC to achieve zero-fluoroscopy RVOT VAs mapping. This approach prolongs procedure time and risk of mechanical trauma more than using a non-steerable decapolar diagnostic catheter to map first in our experience. The non-steerable catheter has the advantage of being softer and cheaper than the steerable one, however, manipulation of the non-steerable catheter via IVC to access RVOT during zero-fluoroscopy catheter mapping remains a challenge. The purpose of this study was to demonstrate zerofluoroscopy RFCA for RVOT VAs with a novel approach using the non-steerable decapolar catheter to map RVOT via Superior Vena Cava (SVC).
Materials and Methods
Study population
We conducted a prospective study of all consecutive patients undergoing catheter ablation for RVOT VAs with electrocardiographic features of typical left bundle branch block, inferior axis QRS morphology and a precordial transition ≥ V3 from May 2020 to November 2021. Prior to catheter ablation, the patients underwent laboratory screening such as echocardiography, Electrocardiography (ECG), and 24-hour Holter monitoring to exclude those with structural heart disease and to determine the manifestation of VAs morphology. Anti-arrhythmic drugs were stopped for at least 5 times the half-life before the procedure. All patients provided written informed consent to participate in this study.
Ensite Velocity 3D-Electroanatomic Mapping (EAM) system and Workmate electrophysiological system (St. Jude Medical Company, Irvine, CA, USA) were used for catheter navigation, mapping, and activation mapping. Ablation catheters used were usually bidirectional deflectable with a tip size of 4-5 mm and irrigated or non-irrigated. The fluoroscopy system was used for backup. Catheterization lab personnels who assisted these cases did not wear lead apron at the beginning of each procedure.
RVOT approach and VAs mapping
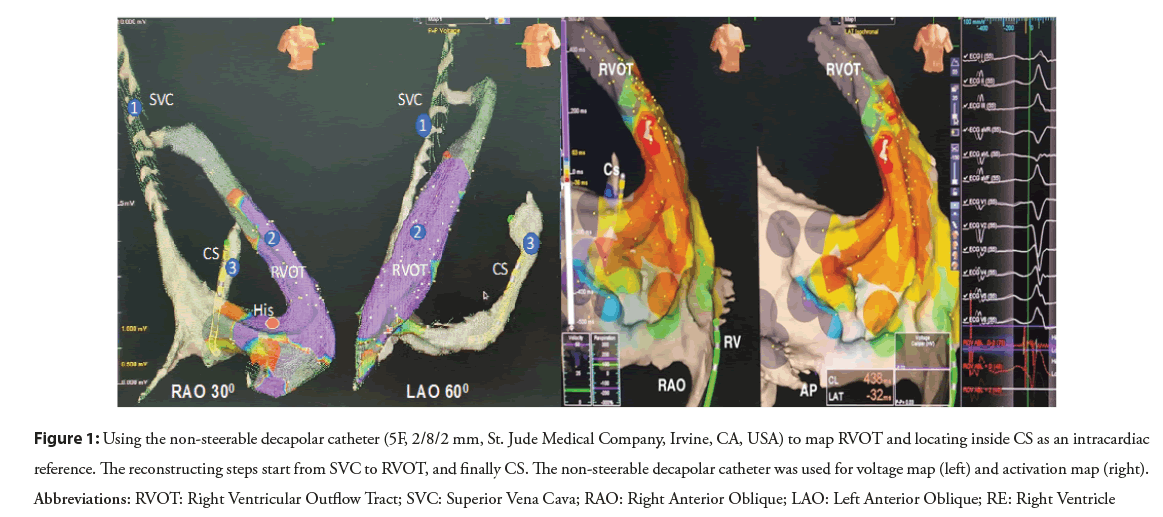
After left subclavian or right jugular vein puncture was confirmed by the venous blood color and the pressure measurement or vascular ultrasound, a 6F (French) sheath was inserted and fixed. The non-steerable decapolar catheter (a decapolar catheter 5F, 2/8/2 mm, St. Jude Medical Company, Irvine, CA, USA) which was reshaped in a form of semicircular curve was connected to the EAM system and external skin patch was used as the reference. Two geometric projections (Left Anterior Oblique (LAO) and Right Anterior Oblique (RAO)) were chosen as a referential navigation for the whole procedure. The decapolar catheter was advanced firstly into Right Atrium (RA) through the SVC and continuosly pushed until the catheter contacted the Tricuspid Annulus (TA). Different sites of catheter tip were confirmed by the obtained intracardiac electrograms. The 3D geometry of SVC and RA was also partly constructed during the advancement of the catheter. As the catheter touched the TA with the intracardiac electrogram received simultaneously both atrial and ventricular signals, the catheter tip was kept perpendicularly and cranially upward in LAO view and was slightly pushed through the TA to RVOT until only ventricular intracardiac electrogram was recorded. The 3D RVOT reconstruction and electro-anatomic activation map were simultaneously performed to record the earliest local activation during PVC or VT. At the area of earliest local activation, the pace map could be utilized to select the target ablation site with the similarity of the 12-lead QRS morphology between paced and clinical VPCs with at least 11 of 12 leads matching. After the completion of reconstruction and activation mapping, the catheter was pulled back until its distal pair of electrodes was at the TA level. The decapoplar catheter was rotated counterclockwise, directed perpendicularly to the interatrial septum under guidance of both LAO and RAO views. The manipulation was continued until the catheter was inside the coronary vein and the atrioventricular electrograms of the mitral valvular annulus were recorded. The positioned CS decapolar catheter could be to set as an intracardiac reference for mapping confirmation by ablation catheter (Figure 1).
Figure 1: Using the non-steerable decapolar catheter (5F, 2/8/2 mm, St. Jude Medical Company, Irvine, CA, USA) to map RVOT and locating inside CS as an intracardiac reference. The reconstructing steps start from SVC to RVOT, and finally CS. The non-steerable decapolar catheter was used for voltage map (left) and activation map (right). Abbreviations: RVOT: Right Ventricular Outflow Tract; SVC: Superior Vena Cava; RAO: Right Anterior Oblique; LAO: Left Anterior Oblique; RE: Right Ventricle
3D roadmap and ablation
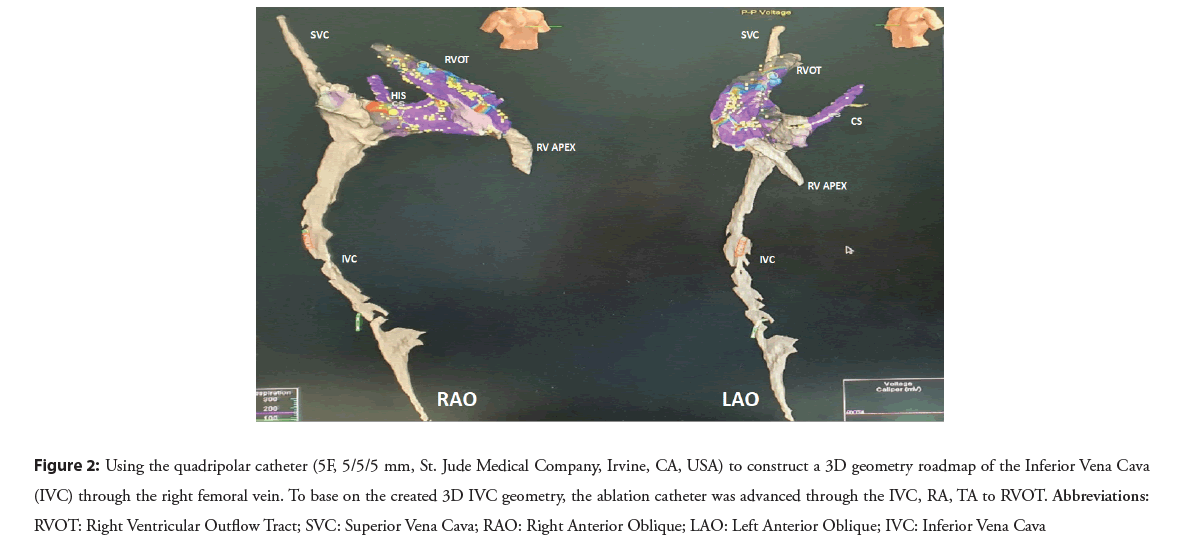
A 3D geometry roadmap of the Inferior Vena Cava (IVC) was reconstructed using a quadripolar catheter (5F, 5/5/5 mm, St. Jude Medical Company, Irvine, CA, USA) through the right femoral vein. The catheter was connected to the monitoring system before insertion and its movement and location were observed using recorded intracardiac electrogram signals on the monitor. The quadripolar catheter was often slightly pushed forward towards the head until it showed the atrial electrogram signal. When it reached the TA, the catheter was advanced towards the 12 o’clock position in LAO view and then placed in the RV apex. The 3D IVC geometry was created by a non-steerable catheter to allow fluoroless approach of ablation catheter to RVOT over the built roadmap.
Based on the created 3D IVC geometry, the ablation catheter after being connected to RF generator was advanced through the IVC, RA, TA to RVOT. If pseudo-geometry of the 3D RVOT model was suspected, the ablation catheter could be used to additionally construct and confirm the action map of interest area (Figure 2).
Figure 2: Using the quadripolar catheter (5F, 5/5/5 mm, St. Jude Medical Company, Irvine, CA, USA) to construct a 3D geometry roadmap of the Inferior Vena Cava (IVC) through the right femoral vein. To base on the created 3D IVC geometry, the ablation catheter was advanced through the IVC, RA, TA to RVOT. Abbreviations: RVOT: Right Ventricular Outflow Tract; SVC: Superior Vena Cava; RAO: Right Anterior Oblique; LAO: Left Anterior Oblique; IVC: Inferior Vena Cava
Radiofrequency energy was delivered at 30-35 W; maximal temperature was not over 60°C for the nonirrigated catheter and 41o C for the irrigated catheter, targeting for an impedance decrease of 10 Ω. After ablation, all patients were followed for 30 minutes in normal condition or under protocol of inducibility or the infusion of isoprenaline.
Statistical analysis
Continuous variables are represented as the mean ± standard deviation. Categorical data are described as percentages. Statistical Package for the Social Sciences (SPSS) version 13.0 (IBM Inc., Armonk, NY) was used to analyze for all data.
Results
Between May 2020 and November 2021, a total of 43 eligible patients underwent RVOT VAs ablation using the 3D road map and were included in this study. We obtained the following results during a mean follow-up of 4.6 months (1-20 months).
Baseline characteristics are presented in Table 1. The mean age was 53.5 (23-83 years old), the female to male rate was 3 to 1, the mean weight was 56.7 ± 7.5 (kg), and mean height was 159.3 ± 5.5 (cm). The most common clinical symptoms were palpitation (72.1%), chest pain (62.8%), while syncope accounted for 11.6%. RVOT VAs were confirmed and classified according to ECG and 24 h Holter recordings. Most of the patients presented with only PVC (79%), while nine patients (20.9%) presented with non-sustained VTs, and only one patient with sustained VT. In addition, echocardiography showed two patients had Left Ventricular Ejection Fraction (LVEF)<50% while other patients had LVEF within the normal range (mean LVEF 63.7 ± 10.76).
| Baseline characteristics of patients | Total (n=43) |
|---|---|
| Age(years) (Mean ± SD) | 53.5 ± 13.2 |
| Sex (female/male) | 31/12 |
| Weight (kg) (Mean ± SD) | 56.7 ± 7.5 |
| Height (cm) (Mean ± SD) | 159.3 ± 5.5 |
| Symptoms | |
| Chest pain (n, %) | 27 (62.8%) |
| Dyspnea (n, %) | 14 (32.6%) |
| Palpitation (n, %) | 31 (72.1%) |
| Syncope/near-syncope (n, %) | 5 (11.6%) |
| Holter recordings | |
| PVC only (n, %) | 34 (79.0%) |
| Nonsustained VT (n, %) | 9 (20,9%) |
| Sustained VT (n, %) | 1 (2.3%) |
| Mean PVC/24 h (%) (Mean ± SD) | 18.4 ± 6.8 |
| ECHO findings | |
| LVEF% (Mean ± SD) | 63.7 ± 10.76 |
Table 1: Demographic and clinical characteristics of the study population.
Table 2 presents electrophysiological features and procedural outcomes. The mapping procedure was implemented by totalzero fluoroscopy in 88.4% of patients and in combination with fluoroscopy in 11.6% of patients to identify the foci of VAs and deliver ablation. Using the 3D road map approach to construct ROVT and confirm the site of VA foci was satisfactory and convenient with a mean mapping time of 27.1 ± 13.31 minutes. RVOT could be divided into 8 regions based on the anatomy [13], in which septal, left, and proximal side (7th site) appeared as the most common foci of VAs by 32.6% and followed by 1st site and 3rd site by 16.3% and 11.6%, respectively. The mean local EAT was 29.28 ± 6.6 ms before deciding to deliver RF to ablate VAs using irrigated catheters (67.4%) or non-irrigated catheters (32,6%) with a total RF time of 494.0 ± 285.9 seconds. After a mean procedural duration of 75.4 minutes (35-180 minutes) with the number of lesions ranging from 1-20, no complication was observed although two patients experienced transient right bundle branch block.
| Characteristics | Values (n=43) |
|---|---|
| Mapping procedure | |
| Total zero-fluoroscopy (n, %) | 38 (88.4%) |
| Switch to combined with fluoro-scopy (n, %) | 5 (11.6%) |
| Ablation catheter | |
| Irrigated (n, %) | 29 (67.4%) |
| Nonirrigated (n, %) | 14 (32.6%) |
| Sites of foci in the RVOT | |
| (1) Free-wall, right, and proximal side (n, %) | 7 (16.3%) |
| (2) Free-wall, right, and distal side (n, %) | 2 (4.7%) |
| (3) Free-wall, left, and proximal side (n, %) | 5 (11.6%) |
| (4) Free-wall, left, and distal side (n, %) | 2 (4.7%) |
| (5) Septal, right, and proximal side (n, %) | 3 (7.0%) |
| (6) Septal, right, and distal side (n, %) | 4 (9.3%) |
| (7) Septal, left, and proximal side (n, %) | 14 (32.6%) |
| (8) Septal, left, and distal side (n, %) | 6 (14%) |
| Procedural results | |
| Total procedure time (minutes) (Mean ± SD) | 75.4 ± 30.0 |
| Mapping time (minutes) (Mean ± SD) | 27.1 ± 13.31 |
| Total RF ablation time (seconds) (Mean ± SD) | 494.0 ± 285.9 |
| Number of lesions (Mean ± SD) | 7.14 ± 4.79 |
| Local EAT (ms) (Mean ± SD) | 29.28 ± 6.6 |
| Major complication (n, %) | 0 (0.0%) |
Table 2: Electrophysiology study and procedural outcomes.
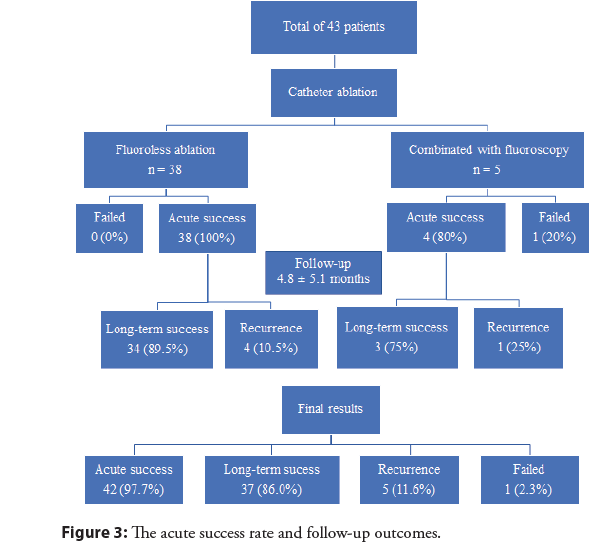
Figure 3 demonstrates acute success rate and follow-up results. All patients underwent an initial approach using zero-fluoroscopy, however, five patients (11.6%) latter required a combination with fluoroscopy. In the group of fluoroless procedure, the acute success rate was 100% in comparison with 80% in the combined with fluoroscopy group. In one patient (2.3%), the procedure failed maybe due to the epicardial site of the VA foci. After a mean follow-up time of 4.8 ± 5.1 months, the long-term success rate of fluoroless ablation and combined ablation were 89.5% and 75%, respectively. Overall, the acute success rate reached 97.7%, the long-term success rate was 86.0%, and the recurrence rate was 11.6%.
Discussion
The main findings
Our study demonstrates that zero-fluoroscopy approach and mapping of RVOT VAs using non-steerable catheter was safe and feasible. Using the non-steerable decapolar diagnostic catheter as a beginning mapping strategy can reduce the procedure time compared with catheter ablation alone in our experience and lowers the cost compared with the addition of the ICE system or a steerable one, while ensuring the equivalent success rate and without causing serious complications.
Previous studies
“As low as reasonably achievable” or ALARA principle is strongly recommended for all electrophysiological procedure to reduce radiation exposure. This approach has been widely performed in RF CA for a variety of arrhythmias.
For a non-fluoroscopic approach in right-sided electrophysiology procedures, Drago and colleagues were the first to report a series of case of catheter ablation performed without fluoroscopy in 2002, including 21 children diagnosed with Wolff-Parkinson-White syndrome with a right-sided accessory pathway. The results showed that 9 cases did not use X-rays at all, while reduced fluoroscopy was achieved in others with an average exposural radiation time of 0.44 minutes. The success rate of the procedure was 20/21 [14]. This study was considered a landmark in the advancement of the field of electrophysiology using the first-generation CARTO system. Since then, the procedure of zero-fluoroscopy ablation was more commonly used under the guidance of the Ensite system [15]. The successful zero fluoroscopy ablation have been reported in several studies. For example, Grubb, et al. using single NavX EAM system, could achieve zero fluoroscopy for 90% of the 76 pediatric patients undergoing right-sided SVT RFCA [16]. Similarly, Ozyilmaz, et al. [11] demonstrated the utility of an EnSite NavX system in the catheter ablation of 17 children with idiopathic ventricular tachycardia, including 9 patients with ventricular tachycardia originating from RVOT. Recently, Zoppo, et al. demonstrated the experience for right-sided arrhythmias in 70 cases from 2013 to 2018 [9]. To our knowledge, most studies focused on atrial arrhythmias and few reports described fluoroscopy reduction in ablation procedures for idiopathic outflow tract VAs.
Our study
For zero-fluoroscopy RVOT VAs ablation, one ablation catheter could be enough to perform both mapping and ablation under the guidance of EAM system. Some authors use a combination of EAM with ICE system to visualize the outflow tract and to completely avoid radiation exposure during the procedure [7,12]. In our opinion, the addition of diagnostic catheters to ablation catheter helps to better localize and represent anatomical markers, thereby increasing the safety of the procedure. In our study, a nonsteerable decapolar catheter (CS catheter) and a quadrupolar catheter were used in addition to the ablation catheter in the same procedure as demonstrated by Wang, et al. [5] and no additional ICE/TEE was used. However, our mapping implementation steps start with using CS catheter to approach RVOT and perform initial activation mappping, the ablation catheter is only used for mapping when it is necessary to verify the 3D structure again. After completing the RVOT mapping, the CS catheter was withdrawn to the right atrium and placed in the CS, making it possible to switch from system reference (external skin patch) to intracardiac reference in situations that more stability is required. Utilization of the CS catheter to map RVOT will collect more points than using a single ablation catheter for the same period of time. Moreover, the non-steerable catheter has the advantage of being softer compared with deflectable catheters, which will reduce the risk of mechanical trauma. However, manipulation of the non-steerable catheter via IVC to access RVOT during zerofluoroscopy catheter mapping remains a challenge and is prone to 3D pseudo-geometry, as well as incomplete activation map in our experience. We change the approach from accessing through SVC to IVC with a fixed CS catheter curve, to not only overcome the above concerns but also make the RVOT access easier. In the study, we used limited fluoroscopy in 5 cases to confirm the ablation catheter position because it was difficult to establish stable contact between the tip of the ablation catheter and the earliest activation site localized by CS catheter.
One of the concerns with this approach is the risk of hemopneumothorax with the use of the subclavian vein. In fact, we usually prefer right jugular vein cannula, or use vascular ultrasound guidance if a subclavian puncture is required. Moreover, most electrophysiology doctors in Vietnam use subclavian vein for CS catheter placement and in our study, no hemopneumothorax was noted.
Using the ICE system or other devices such as contact force catheter, steerable catheter in combination with EAM has been shown to be safe and feasible for fluoroless RFCA of many arrhythmias, particularly with complex procedures like atrial fibrillation RFCA [7,12,17]. They allow direct visualization of tissue contact and stability of the ablation catheter [7]. However, these devices are not readily available in developing countries such as Vietnam and incur significantly higher procedure costs. Therefore, in the context of RVOT arrhythmias, the combination of ablation catheter with non-steerable diagnostic catheters ensures a balance between the benefits of radiation reduction and the cost of the procedure.
Limitations of the Study
This is a single-centre study with small sample size and no control group. Nevertheless, we believe this study provides a safe and costeffective option for non-fluoroscopy RVOT VA ablation, especially in low resources setting where supportive tools such as ICE or steerable catheter are either not available or too expensive. This technique can be widely implemented once its safety and efficacy are confirmed in a randomized controlled trial.
Conclusion
Fluoroless RVOT approach can be performed safely and feasible by using additional non-steerable diagnostic catheter via the SVC. The non-steerable catheter should be used to perform activation mapping of RVOT VAs as the initial step of the procedure. Appropriate utilization of this technique can reduce radiation exposure at an affordable price.
Acknowledgment
The authors thank Cardiovascular center of Hanoi E hospital and Ethics Committee of Hanoi E hospital for the support.
References
- Ector J, Dragusin O, Adriaenssens B, et al. Obesity is a major determinant of radiation dose in patients undergoing pulmonary vein isolation for atrial fibrillation. J Am Coll Cardiol. 50(3): 234-42 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Clay MA, Campbell RM, Strieper M, et al. Long-term risk of fatal malignancy following pediatric radiofrequency ablation. Am J Cardiol. 102(7): 913-5 (2008).
[CrossRef] [Google Scholar] [PubMed]
- Limacher MC, Douglas PS, Germano G, et al. ACC expert consensus document. Radiation safety in the practice of cardiology. American College of Cardiology. J Am Coll Cardiol. 31(4): 892-913 (1998).
- Hindricks G, Willems S, Kautzner J, et al. Effect of electroanatomically guided versus conventional catheter ablation of typical atrial flutter on the fluoroscopy time and resource use: A prospective randomized multicenter study. J Cardiovasc Electrophysiol. 20(7): 734-40 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Wang Y, Chen GZ, Yao Y, et al. Ablation of idiopathic ventricular arrhythmia using zero-fluoroscopy approach with equivalent efficacy and less fatigue: A multicenter comparative study. Medicine (Baltimore). 96(6): e6080 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Akdeniz C, Gul EE, Celik N, et al. Catheter ablation of idiopathic right ventricular arrhythmias in children with limited fluoroscopy. J Interv Card Electrophysiol. 46(3): 355-60 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Lamberti F, Di Clemente F, Remoli R, et al. Catheter ablation of idiopathic ventricular tachycardia without the use of fluoroscopy. Int J Cardiol. 190: 338-43 (2015).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Yamada S, Chung FP, Lin YJ, et al. Electrocardiographic features of failed and recurrent right ventricular outflow tract catheter ablation of idiopathic ventricular arrhythmias. J Cardiovasc Electrophysiol. 29(1): 127-137 (2018).
- Zoppo F, Licciardello C, Favaro G, et al., Safety steps for a non-fluoroscopic approach in right-sided electrophysiology procedures: A point of view. Indian Pacing Electrophysiol J. 19(5): 183-188 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Proietti R, Wang Y, Yao Y, et al. Catheter placement and model reconstruction, in cardiac electrophysiology without fluoroscopy. 45-64 (2019).
- Ozyilmaz I, Ergul Y, Akdeniz C, et al. Catheter ablation of idiopathic ventricular tachycardia in children using the EnSite NavX system with/without fluoroscopy. Cardiol Young. 24(5): 886-92 (2014).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Sánchez JM, Yanics MA, Wilson P, et al. Fluoroless catheter ablation in adults: A single center experience. J Interv Card Electrophysiol. 45(2): 199-207 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Kamakura S, Shimizu W, Matsuo K, et al. Localization of optimal ablation site of idiopathic ventricular tachycardia from right and left ventricular outflow tract by body surface ECG. Circulation. 98(15): 1525-33 (1998).
[CrossRef] [Google Scholar] [PubMed]
- Drago F, Silvetti MS, Di Pino A, et al. Exclusion of fluoroscopy during ablation treatment of right accessory pathway in children. J Cardiovasc Electrophysiol. 13(8): 778-82 (2002).
[CrossRef] [Google Scholar] [PubMed]
- Anderson C, Martinez AR, Razminia M, et al. Zero fluoroscopy ablation: Recent trends in radiation exposure in the EP Lab. Curr Treat Options Pediatr. 5(4): 343-355 (2019).
- Grubb NR, Petzer E, Lang C, et al. A zero fluoroscopy approach for electrophysiologic studies and catheter ablation for common supraventricular tachycardias. Heart Rhythm. 3(5): S123 (2006).
- Razminia M, Willoughby MC, Demo H, et al. Fluoroless catheter ablation of cardiac arrhythmias: A 5-year experience. Pacing Clin Electrophysiol. 40(4): 425-433 (2017).
[CrossRef] [Google Scholar] (All versions) [PubMed]